
�����( 24��)��1��25�桢101 kPa�£�1 mol ����ȼ������Һ̬ˮ���ų�285.8kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ___________________________________________________��
��2�� 25�棬101kPaʱ��16 g CH4(g)������O2(g)��Ӧ����CO2(g)��H2O(l)���ų�890.3 kJ��������CH4ȼ�յ��Ȼ�ѧ����ʽΪ_______________________________________________��
��3��25�棬101kPaʱ��0.5 mol CO��������O2�г��ȼ�գ��ų�141.3 kJ���ȣ���CO��ȼ����Ϊ �����ʾȼ���ȵ��Ȼ�ѧ����ʽ�� ��
��4��0.50L 2.00mol/L H2SO4��2.00L 1.00mol/L KOH��Һ��ȫ��Ӧ���ų�114.6kJ���������÷�Ӧ���к���Ϊ �����ʾ�к��ȵ��Ȼ�ѧ����ʽΪ ��
��5����֪��1molH-H����1molN-H����1molN��N���ֱ���Ҫ��������436kJ��391 kJ��946 kJ����25�棬101kPaʱ��N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽ�� ��
��1��2H2(g)+O2(g)=2H2O(l) ��H= -571.6 kJ/mol
��2�� CH4(g)+2O2(g)=2H2O(l) +CO2(g) ��H= -890.3 kJ/mol
��3�� 282.6 kJ/mol��CO (g)+1/2O2(g)=CO2(l) ��H����282.6 kJ/mol
��4�� 57.8 kJ��mol��1 1/2H2SO4(aq)��1/2NaOH(aq)=== 1/2Na2SO4(aq)��H2O(l) ��H����57.8 kJ��mol��1
��5�� N2(g)��3H2(g)?===?2NH3(g) ��H����92 kJ��mol��1
��������
�����������1��25�桢101 kPa�£�1 mol ����ȼ������Һ̬ˮ���ų�285.8kJ������������ȼ�յ��Ȼ�ѧ����ʽΪH2(g)+1/2O2(g)��H2O(l) ��H����285.8kJ/mol��
��2��25�棬101kPaʱ��16 g CH4(g)��1mol����������O2(g)��Ӧ����CO2(g)��H2O(l)���ų�890.3 kJ��������CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4(g)+2O2(g)��2H2O(l) +CO2(g) ��H= -890.3 kJ/mol��
��3��ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������25�棬101kPaʱ��0.5 mol CO��������O2�г��ȼ�գ��ų�141.3 kJ����������1molCO��������O2�г��ȼ�գ��ų�2��141.3 kJ��282.6������������CO��ȼ����Ϊ282.6 kJ/mol�����ʾȼ���ȵ��Ȼ�ѧ����ʽ��CO (g)+1/2O2(g)=CO2(l) ��H����282.6 kJ/mol��
��4���к�������һ�������£�ϡ��Һ�У�ǿ���ǿ�Ӧ����1molˮʱ���ų���������0.50L 2.00mol/L H2SO4��2.00L 1.00mol/L KOH��Һ��ȫ��Ӧ���ų�114.6kJ����������������ˮ�����ʵ�����2mol����������1molˮ�ų���������114.6kJ��2��57.8����˸÷�Ӧ���к���Ϊ57.8 kJ��mol��1�����ʾ�к��ȵ��Ȼ�ѧ����ʽΪ1/2H2SO4(aq)��1/2NaOH(aq)��1/2Na2SO4(aq)��H2O(l)��H����57.8 kJ��mol��1��
��5���ڷ�ӦN2+3H2 2NH3�У�����3molH-H����1molN��N�������յ�����Ϊ3��436kJ+946kJ��2254kJ������2molNH3�����γ�6molN-H�����ų�������Ϊ6��391kJ��2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������Ϊ2346kJ-2254kJ��92kJ��N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��
2NH3�У�����3molH-H����1molN��N�������յ�����Ϊ3��436kJ+946kJ��2254kJ������2molNH3�����γ�6molN-H�����ų�������Ϊ6��391kJ��2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������Ϊ2346kJ-2254kJ��92kJ��N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g�� 2NH3��g����H����92kJ?mol-1��
2NH3��g����H����92kJ?mol-1��
���㣺���鷴Ӧ�ȵ��й��жϡ������Լ��Ȼ�ѧ����ʽ����д


 ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫�����صڶ���ѧ�߶�10���¿���ѧ�������Ծ��������棩 ���ͣ�ѡ����
ˮ��Һ���ܴ��������һ��������( )
A��Na+��OH-��Cl-��HCO3- B. H+��Na+��Fe2+��MnO4-
C��K+��Ca2+��Cl-��NO3- D. K+��NH4+��OH-��SO42-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶�10���¿���ѧ���ģ��Ծ��������棩 ���ͣ�ѡ����
���������У������ںϽ����( )
A. Ӳ�� B. ��ͭ C. ˮ�� D. ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶�10���¿���ѧ���ģ��Ծ��������棩 ���ͣ�ѡ����
�����������ڵ��ʵ��ǣ� ��
A��Һ�� B����ˮ C��Ư��Һ D��Ư��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶���ѧ�ڵ�һ���¿����ƻ�ѧ���������棩 ���ͣ�������
(8��)�����£���a mol N2��b mol H2�Ļ������ͨ��һ���ݻ�Ϊ2L���ܱ������У��������·�Ӧ�� N2 (g) + 3 H2(g)  2NH3(g)������Ӧ���е�5minʱ�����n (N2) = 13mol��n (NH3) = 6mol������aֵ��N2����ʼŨ�ȼ���H2��ʾ�ķ�Ӧ����(Ҫ�м������)��
2NH3(g)������Ӧ���е�5minʱ�����n (N2) = 13mol��n (NH3) = 6mol������aֵ��N2����ʼŨ�ȼ���H2��ʾ�ķ�Ӧ����(Ҫ�м������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶���ѧ�ڵ�һ���¿����ƻ�ѧ���������棩 ���ͣ�ѡ����
һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ������������ȷ����
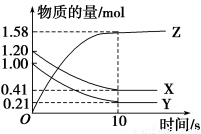
A����Ӧ��ʼ��10 sĩʱ����Z��ʾ�ķ�Ӧ����Ϊ0.158 mol��L��1��s��1
B����Ӧ��ʼ��10 sĩʱ��X�����ʵ���Ũ�ȼ�����0.79 mol��L��1
C����Ӧ��ʼ��10 sʱ����Y��ʾ�ķ�Ӧ����Ϊ0.0395mol��L��1��s��1
D����Ӧ�Ļ�ѧ����ʽΪX(g)��Y(g)��2Z(g)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶���ѧ�ڵ�һ���¿����ƻ�ѧ���������棩 ���ͣ�ѡ����
����˵������ȷ����
A����ѧ��Ӧ���������������⣬�������������ı仯
B�����ڦ�H>0�ķ�Ӧ����Ӧ�������С�������������
C�����ȷ�Ӧ������Ҫ���Ⱦ��ܷ���
D�����ȷ�Ӧ��һ������(�糣�¡����ȵ�)��Ҳ�ܷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶���ѧ�ڵ�һ���¿��Ŀƻ�ѧ���������棩 ���ͣ�ѡ����
���������ദ�������õı�־�У���ͼ����
A������������־
B��Σ�շ����־
C���ɻ������־
D������������־
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ��㶫ʡ�߶���ѧ�ڵ�һ���¿��Ŀƻ�ѧ���������棩 ���ͣ�ѡ����
�� 28g KOH ����ˮ���Ƴ� 1L ��Һ, �� KOH��Һ�����ʵ���Ũ����
A�� 0.5mol/ L B�� 1mol/ L C�� 2mol/ L D�� 4mol/ L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com