


 ПьРжаЁВЉЪПЙЎЙЬгыЬсИпЯЕСаД№АИ
ПьРжаЁВЉЪПЙЎЙЬгыЬсИпЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
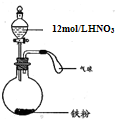 ЃЈ2009?ЫЩНЧјЖўФЃЃЉШчЭМЫљЪОЃЌЯђвЛЖЈСПЕФЬњЗлжаМгШывЛЖЈЬхЛ§12mol/LЕФЯѕЫсЃЌМгШШГфЗжЗДгІКѓЃЌЯТСаЮЂСЃдкЬхЯЕжаПЩФмДѓСПДцдкЕФЪЧЃЈЁЁЁЁЃЉ
ЃЈ2009?ЫЩНЧјЖўФЃЃЉШчЭМЫљЪОЃЌЯђвЛЖЈСПЕФЬњЗлжаМгШывЛЖЈЬхЛ§12mol/LЕФЯѕЫсЃЌМгШШГфЗжЗДгІКѓЃЌЯТСаЮЂСЃдкЬхЯЕжаПЩФмДѓСПДцдкЕФЪЧЃЈЁЁЁЁЃЉВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com