
- 3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| T/��K�� | 298 | 398 | 498 | �� |
| K/��mol?L-1��-2 | 4.1��106 | K1 | K2 | �� |
2- 4 |
+ 4 |
+ 4 |
2- 4 |
+ 4 |
2- 4 |
+ 4 |
2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
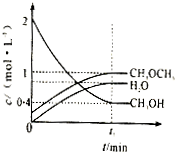 ��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ��
��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�����д����߶���ѧ(�²�)��������ϩ����Ȳ�� ���ͣ�058
ʵ�����Ƽ���ķ����ǽ���ˮ������(![]() )�ͼ�ʯ�ҵĻ�����ǿ�ȣ���Ӧԭ��Ϊ��
)�ͼ�ʯ�ҵĻ�����ǿ�ȣ���Ӧԭ��Ϊ��
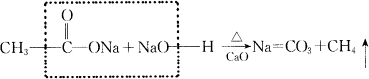
(1)�ӷ�Ӧ��ʹ�õ�ҩƷ�ͷ�Ӧ����������ʵ������![]() ��װ��Ӧ���Ʊ�________��________�����װ����ͬ��
��װ��Ӧ���Ʊ�________��________�����װ����ͬ��
(2)�ռ�![]() ����ɲ���________��________������
����ɲ���________��________������
(3)��ʵ���б���ʹ����ˮ![]() ��������ʹ��
��������ʹ��![]() ���壬���ò���
���壬���ò���![]() ���壮�Դӷ�Ӧ�л�ѧ�����ѵķ�ʽ�������ܵ�ԭ��________��
���壮�Դӷ�Ӧ�л�ѧ�����ѵķ�ʽ�������ܵ�ԭ��________��
(4)��֪����ͬ������ʹ������������Ҳ�ɷ������Ƶķ�Ӧ��������з�Ӧ�Ļ�ѧ����ʽ��
��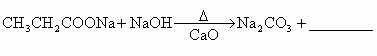 ��
��
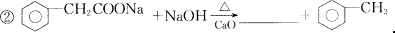
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ����һ�и���5��ģ�⿼�������ۺϻ�ѧ�Ծ����������� ���ͣ������
��14�֣���֪A��B��C��D��EΪ��ѧ��ѧ�������������ʣ�����Ԫ��R��R��A��B��C��D��E�����ʻ��ϼ����ε���������ֻ��BΪ���ʡ������£�A��B��C��DΪ���壬��D+H2O��C+E��
��ش��������⣺
��Ԫ��R��Ԫ�����ڱ��е�λ��Ϊ_________ ____�� B���ӵĵ���ʽΪ__________��
����֪����D��NaOH��Һ1:1ǡ����ȫ��Ӧ������R�����ֺ������Σ�������Һ�и�����Ũ�ȴ�С��ϵ ��
�ǽ�22.4LijR��������������������ͭ����ȫ��Ӧ�����������Ϊ11.2L�����������ͬ�����²ⶨ�������������Ļ�ѧʽ����Ϊ ��������ţ�
�١�RO2 �ڡ�R2O3 �ۡ�RO �ܡ�R2O
�ȿ�ѧ���Ʊ�����һ�ֻ������A�����Ԫ����ͬ�����кܸߵ���ֵ�������������ȼ�ϵ�ص�ȼ�ϡ��û�������ɴ���������Һ��A��Ӧ�õ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��MΪE�����Σ�һ�������¿ɷ������·�Ӧ��
�����ʵ�鷽����������������N ��
�ڴ���,��ѧ�һ��Ʊ�����һ������X������P�����Ԫ����ȫ��ͬ��X�е���������P�е������ӱ�����ʽ��ͬ��Ԫ�������ԭ�Ӹ�������ͬ������X�������ӵĽṹ�к���һ����������-O-O- ���絼ʵ�������ͬ��������絼������NaCl��ͬ������X����������ˮ��Ӧ���ɹ�����������ӷ���ʽΪ ��
| 25��ƽ����ϵ������ˮ��HA�� | ƽ�ⳣ�� | �ʱ� | ��ʼ��Ũ�� |
��ˮ�У�HA H����A�� H����A�� | K1 | ��H1 | 3.0��10��3 mol��L��1 |
�ڱ��У�2HA (HA)2 (HA)2 | K2 | ��H2 | 4.0��10��3 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����5��ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��14�֣���֪A��B��C��D��EΪ��ѧ��ѧ�������������ʣ�����Ԫ��R��R��A��B��C��D��E�����ʻ��ϼ����ε���������ֻ��BΪ���ʡ������£�A��B��C��DΪ���壬��D+H2O��C+E��
��ش��������⣺
��Ԫ��R��Ԫ�����ڱ��е�λ��Ϊ_________ ____�� B���ӵĵ���ʽΪ__________��
����֪����D��NaOH��Һ1:1ǡ����ȫ��Ӧ������R�����ֺ������Σ�������Һ�и�����Ũ�ȴ�С��ϵ ��
�ǽ�22.4LijR��������������������ͭ����ȫ��Ӧ�����������Ϊ11.2L�����������ͬ�����²ⶨ�������������Ļ�ѧʽ����Ϊ ��������ţ�
�١�RO2 �ڡ�R2O3 �ۡ�RO �ܡ�R2O
�ȿ�ѧ���Ʊ�����һ�ֻ������A�����Ԫ����ͬ�����кܸߵ���ֵ�������������ȼ�ϵ�ص�ȼ�ϡ��û�������ɴ���������Һ��A��Ӧ�õ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��MΪE�����Σ�һ�������¿ɷ������·�Ӧ��

�����ʵ�鷽����������������N ��
�ڴ���,��ѧ�һ��Ʊ�����һ������X������P�����Ԫ����ȫ��ͬ��X�е���������P�е������ӱ�����ʽ��ͬ��Ԫ�������ԭ�Ӹ�������ͬ������X�������ӵĽṹ�к���һ����������-O-O- ���絼ʵ�������ͬ��������絼������NaCl��ͬ������X����������ˮ��Ӧ���ɹ�����������ӷ���ʽΪ ��
|
25��ƽ����ϵ������ˮ��HA�� |
ƽ�ⳣ�� |
�ʱ� |
��ʼ��Ũ�� |
|
��ˮ�У�HA |
K1 |
��H1 |
3.0��10��3 mol��L��1 |
|
�ڱ��У�2HA |
K2 |
��H2 |
4.0��10��3 mol��L��1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com