
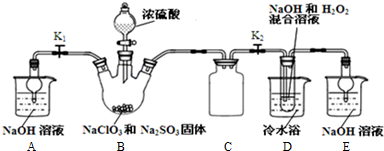
���� װ��B���Ʊ��õ�ClO2������B�з�ӦΪNaClO3��Na2SO3��ŨH2SO4���������� ClO2��Na2SO4���������Ⱥ��������Ʒ�Ӧ����NaClO2��B�п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 ������D�У�SO2��H2O2 ��Ӧ���������ƣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl��װ��C�������ǰ�ȫƿ���з��������ã���װ��D����Һ���NaClO2���壬��Ҫ�����ᾧ�����ȹ��ˡ�ϴ�ӡ����װ��AE�����ն��������ֹ��Ⱦ��
��1����50%˫��ˮ����30%��H2O2��Һ����Ҫ����������Ͳ���ձ���������������ιܣ�
װ��D�з������巴Ӧ��װ����ѹǿ���ͣ�װ��C�������ǰ�ȫƿ����ֹDƿ��Һ������Bƿ�У�
��2��װ��B���Ʊ��õ�ClO2��װ��D��Ӧ�����Һ���NaClO2���壬װ��D������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��д����ʽ��B�Ƶõ������к���SO2����װ��D�б������������ᣬ�������������ᱵ�ǰ�ɫ���������������B�п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 ������D�У�SO2��H2O2 ��Ӧ���������ƣ�
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����ע���¶ȿ��ƣ�
��4������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl��
��5���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
�ڸ��ݻ�ѧ��Ӧ�ɵù�ϵʽ��NaClO2��2I2��4S2O32-������Ʒ��NaClO2�����ʵ���x�����ݹ�ϵʽ���㣮
��� �⣺��1����50%˫��ˮ����30%��H2O2��Һ����Ҫ����������Ͳ���ձ���������������ιܣ����Ի���Ҫ��Ͳ��
װ��D�з������巴Ӧ��װ����ѹǿ���ͣ�װ��C�������ǰ�ȫƿ����ֹDƿ��Һ������Bƿ�У�
�ʴ�Ϊ����Ͳ����ֹDƿ��Һ������Bƿ�У�
��2��װ��B���Ʊ��õ�ClO2��װ��D��Ӧ�����Һ���NaClO2���壬װ��D������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��ʽΪ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��
B�Ƶõ������к���SO2����װ��D�б������������ᣬ��Һ�п��ܴ���SO42-�����Ȼ�����Һ����SO42-�����������ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-��B�п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 ������D�У�SO2��H2O2 ��Ӧ���������ƣ�Ũ�����ѻӷ������������ѻӷ����Σ��������D����a��ȷ��b��c����
��ѡ��a��
�ʴ�Ϊ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��SO42-��ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42-��a��
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO2•3H2O��Ӧ���ȹ��ˣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�����ϴ�ӣ�����60����
�ʴ�Ϊ����38�桫60����ˮϴ�ӣ�����60����
��4������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl�����������ȥD�е���ˮԡ�����ܵ��²�Ʒ�л��е�������NaClO3��NaCl��
�ʴ�Ϊ��NaClO3��NaCl��
��5���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣬
�ʴ�Ϊ���μ����һ��Na2S2O3��Һʱ����Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
������Ʒ��NaClO2�����ʵ���x����
NaClO2��2I2��4S2O32-��
1mol 4mol
0.25x c mol•L-1��V��10-3L
��x=c•V•10-3mol
�ʴ�Ϊ��c•V•10-3mol��
���� ���⿼�����������Ʊ�ʵ��Ļ����������������Ƶ����ʼ��к͵ζ���֪ʶ������ԭ���ǽ���Ĺؼ���ͬʱ����ѧ���������⡢����������������Ŀ�Ѷ��еȣ�


 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ����ܶ� | �۵㣨�棩 | �е㣨�棩 | �ܽ�� | |
| ˮ | ���� | ||||
| ����ȩ | 1.04 | -26 | 179.6 | �� | ���� |
| ������ | 1.27 | 122.1 | 249 | 25���ܣ�95����� | ���� |
| ���״� | 1.04 | -15.3 | 205.7 | �� | ���� |
| ���� | 0.71 | -116.3 | 34.6 | ���� | -- |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | ���ڵ��������� | C�� | ʳ��ˮ | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 N2+4NH3��Ϊ�����µķֽ⣬�ɲ�ȡ�ĺ�����ʩ�н��ͷ�Ӧ�¶ȣ���дһ�֣���
N2+4NH3��Ϊ�����µķֽ⣬�ɲ�ȡ�ĺ�����ʩ�н��ͷ�Ӧ�¶ȣ���дһ�֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AlCl3��Һ���ռ���Һ��Ӧ����n��OH-����n��Al3+��=7��2ʱ��2Al3++7OH-�TAl��OH��3��+AlO2-+2H2O | |
| B�� | CuCl2��Һ��NaHS��Һ��Ӧ����n��CuCl2����n��NaHS��=1��2ʱ��Cu2++2HS-�TCuS��+H2S�� | |
| C�� | Cl2��FeBr2��Һ��Ӧ����n��Cl2����n��FeBr2��=1��1ʱ��2Fe2++4Br-+3Cl2�T2Fe3++2Br2+6Cl- | |
| D�� | Fe��ϡ���ᷴӦ����n��Fe����n��HNO3��=1��2ʱ��3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com