
Ϊ���о�ij������Ʒ����
���ԣ�ijͬѧ25��ʱ��������ʵ�飺
��ȡm g�������ձ��У�����VmL������ˮ����ֽ��裬���ú���ˣ��ⶨ��Һ��pH��
������Һ�еμӰ�ˮ��ÿ����2 mL��ˮ�ͽ�����Ȳ�������Һ��pH��
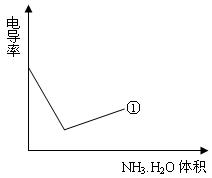
��������ʵ���¼,�Լ���İ�ˮ���Ϊ�����ꡢpHΪ�������������ͼ���£�

����������¼�����ݣ��ش��������⣺
��1�������������������ԣ����ᡢ��У�
��2����֪25��ʱ, ���ð�ˮ��Ũ��= 6.0??10-4mol/L����ˮ�ĵ��볣��K = ��
Ϊʹ����Һ�����ԣ������백ˮ��ǡ������� ��
��4���絼���Ǻ����������Һ������
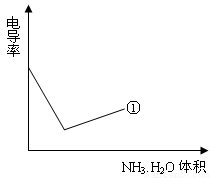
����С����������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣����ʾ��ͼ�У����ߢ�����NH3��H2O��Һ�ζ�HCl��Һʱ���μ�NH3��H2O��Һ�������Һ�絼�ʵĹ�ϵ����������ڸ�ͼ�л�����NH3��H2O��Һ�ζ�CH3COOH��Һʱ�����ߡ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�Ը�����������������⣺
����һ��������Ӣ����ѧ��R.A.Smith 1872����������ģ�һ����ָpHС��5.6���ꡢѩ�����ȴ�����ˮ���Ǵ�����Ⱦ�ı��֡����걻����������������ij����1998�껷��״�������أ���������Ƶ��Ϊ41.3����������������5.5���ٷֵ㡣��ˮ����ƽ��pHΪ4.87����ˮ������Ⱦ�����أ�����Ϊú������Ⱦ������
���϶�����ij��һ���ϴ�Χ������ʹֲ��Ҷ�����ʴ�����������ߵ���������������ϴ������������ʧ���ء�������ʹֲ�������Ӵ�Ҷ��������ʹ�ơ�þ����������Ѹ�ٴ���������ʧ������Ӫ��״�����ͣ���ʹ��������ܵ����ƣ�Ӱ��ֲ���������������������˵���к�Ԫ�أ�������������������ʽ���ڣ�pH=5.6ʱ�������ܽ⡣��pHΪ4.6ʱ�������ܽ������100������ת�����������������ľ�������Ĵ�ij�ֳ�����ɽ�������ʴ�96������������ʹ������������Ũ�����ߣ�Σ����ɽ�ɡ�
���������ҹ�ũҵ����������������ɵ���ʧÿ��ߴ�15��Ԫ��Ϊ����Ч�������꣬����Ժ�Ѿ����ˡ�����������Ͷ���������������ַ������ȹ涨��
ij�о���ѧϰС��ƻ��о�����������γɹ��̣�����ȡ����ˮ��ˮ�����вⶨ����ʱ������ƣ���βⶨ����Ʒ��pH���õ��������ݣ�
| ʱ��/h | ��ʼ | 8 | 16 | 24 | 32 | 40 | 48 |
| pH | 5.0 | 4.8 | 4.5 | 4.3 | 4.2 | 4.0 | 4.0 |
���⣺����ˮ��Ʒ����ʱpH�仯����Ҫԭ��(�û�ѧ����ʽ��ʾ)_____________________���ɴ˿��Եó�������γɹ��̿�����
______________________________________________��
���������ȡ����������ˮ������ˮ���ϣ�pH��_________________ (�������С�����䡱)��ԭ����__________________________________�������������_____________�ԡ�
(2)�о������������ɵ�Σ����
(3)����Ϊ�������������;���ɲ�ȡ�����д�ʩ��
__________________________________��
������ú��ȼ�� �ڰѹ������̴���� ��ȼ������ �������ữ�������м�ʯ�� �ݿ�������Դ
A.�٢� B.�ڢۢܢ� C.�٢ۢ� D.�٢ۢܢ�
(4)Ϊ�˼���úȼ��ʱ������ŷŵĶ�������ͬ�ĵط�������ȡ��ͬ�ġ���������
�ٻ������糧����Ca(OH)2������Һ����úȼ��ʱ�����Ķ����������壬�������Ƶ����õ�ʯ��(CaSO4��2H2O)�����йط�Ӧ�Ļ�ѧ����ʽ_____________________��
__________________��___________________��
�����Ṥҵ����Ũ��ˮ�����ն����������йصĻ�ѧ����ʽΪ_____________________��
����Щ��ҵ��ȡ����ʯ�Һͺ���ú���ʹ�á���д��ȼ��ʱ�йء�����Ӧ�Ļ�ѧ����ʽ��
�ܹ��������²��á���������������Һ����SO2��������д���йط�Ӧ�Ļ�ѧ����ʽ��
����Ƚ��������֡�����������ȱ�㡣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�Ϊ���о�ij������Ʒ������ԣ�ijͬѧ25��ʱ��������ʵ�飺
��ȡm g�������ձ��У�����VmL������ˮ����ֽ��裬���ú���ˣ��ⶨ��Һ��pH��
������Һ�еμӰ�ˮ��ÿ����2 mL��ˮ�ͽ�����Ȳ�������Һ��pH��
��������ʵ���¼,�Լ���İ�ˮ���Ϊ�����ꡢpHΪ�������������ͼ���£�
����������¼�����ݣ��ش��������⣺
��1�������������������ԣ����ᡢ��У�
��2����֪25��ʱ, ���ð�ˮ��Ũ��= 6.0Í10-4mol/L����ˮ�ĵ��볣��K = ��
Ϊʹ����Һ�����ԣ������백ˮ��ǡ������� ��
��4���絼���Ǻ����������Һ����������С����������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣����ʾ��ͼ�У����ߢ�����NH3��H2O��Һ�ζ�HCl��Һʱ���μ�NH3��H2O��Һ�������Һ�絼�ʵĹ�ϵ����������ڸ�ͼ�л�����NH3��H2O��Һ�ζ�CH3COOH��Һʱ�����ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������и������ۻ�ѧ���Ծ� ���ͣ�ʵ����
��16�֣�Ϊ���о�ij������Ʒ������ԣ�ijͬѧ25��ʱ��������ʵ�飺��ȡm g�������ձ��У�����VmL������ˮ����ֽ��裬���ú���ˣ��ⶨ��Һ��pH��������Һ�еμӰ�ˮ��ÿ����2 mL��ˮ�ͽ�����Ȳ�������Һ��pH����������ʵ���¼,�Լ���İ�ˮ���Ϊ�����ꡢpHΪ�������������ͼ���£�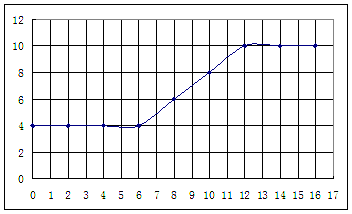
����������¼�����ݣ��ش��������⣺��1�������������������ԣ����ᡢ��У���2����֪25��ʱ, ���ð�ˮ��Ũ��= 6.0Í10-4mol/L����ˮ�ĵ��볣��K = ��Ϊʹ����Һ�����ԣ������백ˮ��ǡ������� ����4���絼���Ǻ����������Һ����������С����������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣����ʾ��ͼ�У����ߢ�����NH3��H2O��Һ�ζ�HCl��Һʱ���μ�NH3��H2O��Һ�������Һ�絼�ʵĹ�ϵ����������ڸ�ͼ�л�����NH3��H2O��Һ�ζ�CH3COOH��Һʱ�����ߡ�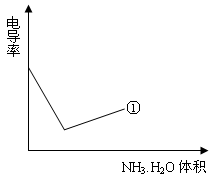
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������и������ۻ�ѧ���Ծ� ���ͣ�ʵ����
��16�֣�Ϊ���о�ij������Ʒ������ԣ�ijͬѧ25��ʱ��������ʵ�飺
��ȡm g�������ձ��У�����VmL������ˮ����ֽ��裬���ú���ˣ��ⶨ��Һ��pH��
������Һ�еμӰ�ˮ��ÿ����2 mL��ˮ�ͽ�����Ȳ�������Һ��pH��
��������ʵ���¼,�Լ���İ�ˮ���Ϊ�����ꡢpHΪ�������������ͼ���£�
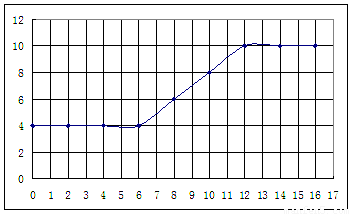
����������¼�����ݣ��ش��������⣺
��1�������������������ԣ����ᡢ��У�
��2����֪25��ʱ, ���ð�ˮ��Ũ��= 6.0Í10-4mol/L����ˮ�ĵ��볣��K = ��
Ϊʹ����Һ�����ԣ������백ˮ��ǡ������� ��
��4���絼���Ǻ����������Һ����������С����������������Һ�絼�ʱ仯����ȷ���ζ���Ӧ���յ㡣����ʾ��ͼ�У����ߢ�����NH3��H2O��Һ�ζ�HCl��Һʱ���μ�NH3��H2O��Һ�������Һ�絼�ʵĹ�ϵ����������ڸ�ͼ�л�����NH3��H2O��Һ�ζ�CH3COOH��Һʱ�����ߡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com