
��ҵ�ϳ��ú�����Al2O3�ķ�����FeO��V2O5�����۷���ȡV2O5����Ҫ�������£�
��֪���ٱ���ʱAl2O3��V2O5�����봿�Ӧ���������Ӧ�����Σ�ͬʱ�ų�CO2����
�ڳ��������ʵ��ܽ�ȣ�NaVO3��21��2g��l00gˮ��HVO3��0��008g/l00gˮ
��ش𣺣�1����д����������ʱ��V2O5�봿�Ӧ��ѧ����ʽ____ ��
��������B������Ҫ�ɷֿ����Ǣ� ����_ ___ ����_ ___����____ ����д��ѧʽ���ɲ�������
��2�������У���ֱ����H2SO4���ݡ�����A����ȡHVO3��ԭ����____ ��
��3���������١����� ��ϴ�ӡ���������ϴ�ӣ����Ʒ�п��ܺ��еĽ����������� �� ������װ�ã����ּг�����ʡȥ��������ʵ���ҽ��С������ڡ�����____ ��������ţ�
��4��NaVO3����ԭ�͵�����������V2O5������NaOH��Һ����ȡ����Ӧ�����ӷ���ʽΪ____ ��
��1��V2O5 +Na2CO3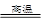 2NaVO3 +CO2 ��2�֣�
2NaVO3 +CO2 ��2�֣�
Fe2O3��Fe3O4������Fe2O3��Fe3O4 ��3�֣�
��2��H2SO4��NaVO3��Ӧ�������ܵ�HVO3��HVO3������һЩ�����ı��棬�谭�˷�Ӧ�Ľ��У��Ӷ�������HVO3���ʣ���ʹ������HVO3���������⣬���������������ᷴӦ���������ӣ����Ӹ������ӣ��������ᣬ�˷���Դ����3�֣�
��3�����ˣ�1�֣�Na+��Al3+��2�֣�B ��2�֣�
��4��V2O5 +2OH�� 2VO3��+ H2O ��2�֣�
2VO3��+ H2O ��2�֣�
���������������1������V2O5���봿�Ӧ���������Ӧ�����Σ�ͬʱ�ų�CO2���壬���仯ѧ����ʽΪV2O5 +Na2CO3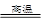 2NaVO3 +CO2���ڱ��յĹ�����FeO������ΪFe2O3��Fe3O4�����ԡ�������B������Ҫ�ɷֿ����Ǣ�Fe2O3��Fe3O4����Fe2O3��Fe3O4
2NaVO3 +CO2���ڱ��յĹ�����FeO������ΪFe2O3��Fe3O4�����ԡ�������B������Ҫ�ɷֿ����Ǣ�Fe2O3��Fe3O4����Fe2O3��Fe3O4
��2����ֱ�Ӽ������ᣬ��A�е����������������ᷴӦ���Ӹ������ӣ��������ᣬ�˷���Դ��ͬʱH2SO4��NaVO3��Ӧ�������ܵ�HVO3��HVO3������һЩ�����ı��棬�谭�˷�Ӧ�Ľ��У��Ӷ�������HVO3���ʣ���ʹ������HVO3������
��3�����������������ܵ�HVO3����������˳������ù�����������ƫ�����Ʒ�Ӧ�������������ӣ���������ϴ�ӳ���������ܺ���Na+��Al3+���������ǰ�HVO3�������ȷֽ��V2O5������Ӧѡ��Bװ�ã�
��4��V2O5������NaOH��Һ�У�����֮һ��NaVO3�������Ʋ���һ������ˮ�����Է�Ӧ�����ӷ���ʽΪ
V2O5 +2OH�� 2VO3��+ H2O
2VO3��+ H2O
���㣺���黯ѧ�л�ѧ����ʽ�����ӷ���ʽ����д����ѧ��Ӧ������жϡ�������ʵ�鲽�衢װ�õ��ж�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ƹ㷺����ʳƷ��������ʯ�͵ȹ�ҵ�����ϣ�300��400�����ҷֽ⡣��ʵ������ȡ�ķ���֮һ�ǣ�Ca(OH)2 +2HCHO + H2O2 = Ca(HCOO)2 + 2H2O + H2����
ʵ������ȡʱ������ҵ���������ƺͼ�ȩ���μ��뵽��������Ϊ30-70%�Ĺ���������Һ�У�Ͷ�����ʵ���֮������Ϊ1��2��1.2�������տɵõ���������98%�������ؽ����������͵����ʲ�Ʒ��
��1��������������������Զ࣬��Ŀ���� ��
��2����Ӧ�¶���ÿ�����30-70��֮�䣬�¶Ȳ����ߣ�����Ҫԭ���� ��
��3���Ʊ�ʱ�ڻ����Һ��Ҫ���������������Ƽ�ȩ��������Ӧ�⣬��Ҫ����������Na2S��Һ�������Ƶ�Ŀ���� ��
��4��ʵ��ʱ��ǿ������45min����Ŀ���� ���������������Һ��pH 7��8����Ŀ���� ����ᾧ���롢����ò�Ʒ��
��ij�о���ѧϰС���ù�ҵ̼���(��Ҫ�ɷ�ΪCaCO3������Ϊ��Al2O3��FeCO3) Ϊԭ�ϣ����Ʊ������Σ������������Һ�����ȡ����ơ������ͼ�������ʵ��ܽ������������ؽ������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0 mol��L-1����)�����ṩ���Լ��У�a.�����ƣ�b.5mol��L��1���ᣬc. 5mol��L��1���ᣬd. 5mol��L��1���ᣬe. 3%H2O2��Һ��f.����ʯ��ˮ��
�벹��������̼����Ʊ�����Ƶ�ʵ�鲽��
| ���� ���� | ��ʼ���� ��pH | ������ȫ ��pH |
| Fe3�� | 1. 1 | 3. 2 |
| Al3�� | 3. 0 | 5. 0 |
| Fe2�� | 5. 8 | 8. 8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��ʵ�����ɴ�����ͭм�Ƶ�����CuSO4��5H2O����ʵ���������£�
�ܽ�ͭмһ�ַ����ǣ���ͭм���뵽ϡ������˫��ˮ�Ļ��Һ�в���30��40��ˮԡ���ȣ�һ��ʱ���ͭ��ȫ�ܽ⣬�õ�����ͭ��Һ��
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ ��
�ڷ�Ӧ�¶Ȳ��ܳ���40���ԭ���� ��
��������ͭ��Һ��õ����IJ�������Ϊ ����ȴ�ᾧ�� ��ϴ�ӡ����
��2��Ŀǰ�ҹ��Ļ����������������Ϊȼú���飬����ȼúΪ���ĵ�����������ɵĻ�����Ⱦ����Լ������ҵ��չ��һ����Ҫ���أ����е������NOx���Ǽ̷۳��Ͷ�������֮��ȼú��վ�����������ص㡣
��ȼú��������ķ����ܶ࣬��ʯ��ʯ��ʯ�෨����ˮ���ȡ�����ʯ��ʯ-ʯ�෨�����ԭ����һ����SO2+Ca(OH)2=CaSO3+H2O��Ȼ���ٽ����������Ƴ�ʯ��,д���÷�Ӧ�Ļ�ѧ����ʽ ��
��ȼú���������ɲ��ð���NH3����Ϊ��ԭ���ʣ��ڴ������ڵ������£���������(NOx)�뻹ԭ��������Ӧ���������ĵ�����ˮ��д�����������백��Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ҵ�������ų������ı���[��Ҫ����BaCO3��BaSiO3��BaSO3��Ba��FeO2��2��]��ij��Ҫ����BaCl2��BaCO3��BaSO4�Ļ��������ñ�����ȡBa��NO3��2���䲿�ֹ����������£�
��1�����ܺ���Һ��pH��1��Ba��FeO2��2��HNO3��Ӧ�Ļ�ѧ����ʽΪ________��
��2������ʱͨ�����Ʒ�Ӧ�¶Ȳ�����70 �棬�Ҳ�ʹ��Ũ���ᣬԭ����________��________��
��3���ó���ϱ���ʵ�ʣ�ѡ�õ�XΪ________���ѧʽ�����к͢�ʹ��Һ��________�������ӷ��ţ���Ũ�ȼ�С���к͢��������Һ����仯�ɺ��ԣ���
��4������������ϴ�ӵ�Ŀ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijθҩ����Ҫ�ɷ���˫�ǻ���̼����[NaAl(OH)2CO3]����������θ����ࡣ
�����ȷ�ϸ�ҩ���к���NaԪ�أ�
_________________________________________________________________��
�����ȷ�ϸ�ҩ���к���AlԪ�غ�CO32����
_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���С�����Ԫ�ء�֮�ƣ���ˮ���庬��Ϊ65 mg��L��1��ʵ����ģ�⺣ˮ�������ȡ��
ʵ�鷽����������������ˮ���뵽20 mL���������ӵĺ�ˮ��(��ˮ������0.1 mol��L��1��NaBr��Һ����)�������û���������ˮ�е�������________________�����漰��Ӧ�����ӷ���ʽΪ__________________��
�õ��ĵ�����������л��ܼ���________(����)��ʵ����ȡ��������ȡ�Լ���________�����ȣ��õ����л�����________ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ͼɼ���п�̸ɵ���ڲ��ĺ�ɫ����A��Ҫ����MnO2��NH4CI��ZnCI2������������FeCI2��̿�ۣ���A�Ʊ��ߴ�MnCO3��������ͼ���¡�
��1������п�̸ɵ�صĸ���������_________(�ѧʽ)��
��2���ڢ�����Ŀ����________________________��
��3���ڢ��������Ƕ���Һa������ȳ��ӣ���ȥZn2+�����ӷ���ʽΪ____________________�� (��֪��Ksp(MnS)=2.5��10-13��Ksp(ZnS)=1.6��10-24)
��4��Ϊѡ���Լ�X������ͬ�����£��ֱ���5 g��ɫ����M�����Ʊ�MnSO4��ʵ�飬�õ��������ұ���
���Լ�x�����ѡ����_________��
�ڵڢ�����ѡ��������Լ�X��M��
��Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽΪ_________��
��5����V��ϵ�в����ɰ��������̽��У�
��֪��MaCO3������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻Mn(OH) 2��
ʼ����ʱpHΪ7.7���벹��������²�����
����1��___________________������2�����ˣ�������ˮϴ��2��3��
����3�������Һ������SO �ѳ��ɾ��� ����4��___________________��
�ѳ��ɾ��� ����4��___________________��
����5�����º�ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ�識�ơ���李����ֳ�±ɰ��Ϊ��ɫ������ɫ�ᾧ�Է�ĩ��������ˮ�У��ڹ�ũҵ��������;�㷺�����Ȼ��ƺ������Ϊԭ���Ʊ��Ȼ�識�����Ʒ�����ƣ������������£�
�Ȼ�狀������Ƶ��ܽ�����¶ȱ仯��ͼ��ʾ���ش��������⣺
��1��ʵ���ҽ�������Ũ���õ�����Ҫ������ ���ձ������������ƾ��Ƶȡ�
��2��ʵ������г��ȹ��˵�Ŀ���� ��
��3��д��������Ũ����ʱ�����Ļ�ѧ����ʽ�� ��
��4��ij�о���ѧϰС��Ϊ�ⶨ��NH4Cl��Ʒ�е��ĺ������������ͼװ�ã������������ۡ�
��ͬѧ�����ݴ�ʵ���õ����ݣ������NH4Cl��Ʒ�ĺ���������ƫ�ߣ���Ϊʵ��װ���д���һ������ȱ���ǣ� ____ ��
��ͬѧ��ʵ������У�����ƿ�м����Ũ����������Һ�����ӷ�Ӧ����ʽΪ ����Ӧ������NaOHһ��Ҫ��������ּ��ȣ�ԭ���� ��
�øĽ����ʵ��װ�����½���ʵ�飬��ȡ13.0gNH4Cl��Ʒ�����ʵ���Bװ������3.4g����û��ʺ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͭ��ʯ��ͭԪ�غ����ϵͣ��Һ�������þ���Ƶ��������ӡ�ijС����ʵ�������ý���-��ȡ���Ʊ�����ͭ��
��1������1������Ϊ ������2�õ��IJ����������ձ���
��2���������������У�Ϊ���ͭ�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ��
��3���Ƚϲ���2֮ǰ�����3֮�����Һ��˵����������ҪĿ���� ��
��4��ȡ����������ҺA���μ� �����������ƣ���Һ��ʺ�ɫ��˵����Һ�д���Fe3+��������Һ�л�����Fe2+�ķ����� ��ע���Լ������������dz�ע������������ʸ��ţ�
��5���õζ����ⶨCuSO4��5H2O�ĺ�����ȡa g�������100 mL��Һ��ȡ20.00mL��c mol /L �ζ���(H2Y2�C���ζ����������ʷ�Ӧ)�ζ����յ㣬���ĵζ���bmL.
�ζ���Ӧ��Cu2+ + H2Y2�C CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ��
CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ��
��6�����в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���_____________��
A���ζ��ٽ��յ�ʱ����ϴƿ�е�����ˮϴ�µζ��ܼ���ڵİ�α�Һ����ƿ��
B���ζ���������ˮϴ�Ӻ�ֱ��ע�����Һ��ȡ20.00mL���еζ�
C���ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com