

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

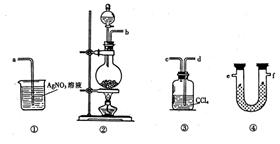
 �����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ�
�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ� �ۣ�____________________
�ۣ�____________________ ��NH4Cl���������NH3��______________(��ܡ����ܡ�)��
��NH4Cl���������NH3��______________(��ܡ����ܡ�)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
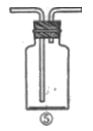
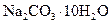 ��������
��������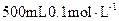 ��
��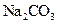 ��Һ����ش��������⡣
��Һ����ش��������⡣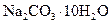 ����_______g��
����_______g��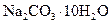 ������__________g��
������__________g��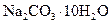 ������ʧȥ���ֽᾧˮ��____________
������ʧȥ���ֽᾧˮ��____________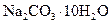 �����л�������
�����л�������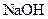 ���壬���������Ƶ�
���壬���������Ƶ�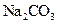 ��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱����
��Һ�����ᷴӦ���ⶨij��������ʵ���Ũ�ȣ����������Ũ�Ȼ�________�����ƫ�ߡ�����ƫ�͡��������䡱�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��12 mol 4 mol | B��6e�� 4e�� | C��12 e�� 4e�� | D��6 mol 4 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƿ��ԭ����������ˮ |
| B����Һ���ձ�ת�Ƶ�����ƿ�к�û��ϴ���ձ� |
| C������ʱ�۲�Һ�温�� |
| D������ʱ��ת����ƿ���Σ����ְ�Һ����͵���ڱ��ߣ��ٲ�����ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B���������� |
| C��������ػ�������� | D������������������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com