
| ʵ���� | HB���ʵ���Ũ��(mol/L) | KOH���ʵ���Ũ��(mol/L) | �����Һ��pH |
| �� | 0.2 | 0.2 | pH��a |
| �� | c1 | 0.2 | pH��7 |
| �� | 0.1 | 0.1 | pH>7 |
| �� | 0.1 | 0.1 | pH��9 |
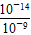 ��10��5(mol/L)���ɵ���غ�ã�c(K��)��c(H��)��c(OH��)��c(B��)����c(Na��)��c(B��)��c(OH��)��c(H��)��(10��5��10��9)mol/L��
��10��5(mol/L)���ɵ���غ�ã�c(K��)��c(H��)��c(OH��)��c(B��)����c(Na��)��c(B��)��c(OH��)��c(H��)��(10��5��10��9)mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 H++HS-��HS-
H++HS-��HS- H++S2-������H2S��Һ��
H++S2-������H2S��Һ�� | A���μ�������ˮ,��ҺpH��С | B��ͨ�����SO2����,��ҺpH���� |
| C����ˮ,��Һ��������Ũ������ | D��������������ͭ����,��������Ũ�ȶ���С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HBr��HCl��BaSO4 |
| B��NH4Cl��CH3COOH��Na2S |
| C��NaOH��Ca(OH)2��NH3��H2O |
| D��HClO��NaF��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| �� | ����ƽ�ⳣ�� |
| ���� | K i=1.75��10-5 |
| ������ | K i=2.98��10-8 |
| ̼�� | Ki1=4.30��10-7 Ki2=5.61��10-11 |
| ������ | Ki1=1.54��10-2 Ki2=1.02��10-7 |
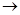 SO32�� + 2HClO
SO32�� + 2HClO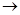 HCO3�� + HClO
HCO3�� + HClO�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��AlCl3��Һ��������AlCl3 |
| B��CH3COONa��Һ���� |
| C����ˮ�м�������NH4Cl���� |
| D��������Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
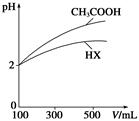
| A�����ǰ��pH(CH3COOH)��pH(NaOH)��14 |
| B����Ϻ�c(Na��)��c(CH3COO��)��c(OH��) |
| C�����ǰ��c(CH3COOH)>c(NaOH) |
| D����Ϻ�c(Na��)>c(CH3COO��)>c(OH��)>c(H��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧʽ | ���볣��(25 ��) |
| HCN | K��4.9��10��10 |
| CH3COOH | K��1.8��10��5 |
| H2CO3 | K1��4.3��10��7��K2��5.6��10��11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����0.1 mol��L��1BA��Һ�У�c(A��)��c(H��)��c(BOH)��c(B��) |
| B������0.1 mol��L��1 BOH��Һϡ����0.001 mol��L��1����Һ��pH��9 |
| C������һ��������������Һ��Ϻ�pH��7������Һ�У�c(A��)>c(B��) |
| D��������������Һ�������1��1��ϣ�����Һ�У�c(A��)>c(B��)>c(H��)>c(OH��) |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com