
����Ŀ�������л������NaCl��NaOH��HCl��FeCl3��CH3COONa��CH3COOH��NH3H2O��H2O
��ش��������⣺
��1��FeCl3��Һ��__________�ԣ������ӷ���ʽ��ʾ______________________��
CH3COOH��Һ��________�ԣ������ӷ���ʽ��ʾ__________________________��
��2�������£�pH=10��CH3COONa��Һ�У�ˮ���������c(OH-)=_________________��
��pH=3HCl����Һ�У�ˮ���������c(H+)=___________________��
��3����֪ˮ��������ƽ�⣺H2O+H2O![]() H3O++OH-��H>0������ʹƽ�������ƶ�����������Һ�����ԣ�ѡ����____________��
H3O++OH-��H>0������ʹƽ�������ƶ�����������Һ�����ԣ�ѡ����____________��
A.��ˮ�м���NaHSO4����
B.��ˮ�м���(NH4)2SO4����
C.������100��[����c(H+)=1��10-6molL-1]
D����ˮ�м�Na2CO3����
��4������Ũ�ȡ�������Ģ�NaOH�͢�NH3H2O�ֱ��ˮϡ��m����n����ϡ�ͺ�������Һ��pH��ȣ���m___________n���<������>����=������
��5��25�棬pH=a������VamL��pH=14-a�İ�ˮVbmL��ϣ�����Һ�����ԣ���Va_____Vb(�>������<������=��������ȷ����)
��6������H2O�⣬������7����Һ�����ʵ���Ũ����ͬ������7����Һ��pH�ɴ�С��˳��Ϊ��______________________________________________������ţ���
��7��������pH=13��NaOH��Һ��pH=2��������Һ��ϣ����û��Һ��pH��11����NaOH������������Ϊ________
���𰸡��� Fe 3+ + 3H2O ![]() Fe��OH��3 + 3H + �� CH3COOH
Fe��OH��3 + 3H + �� CH3COOH ![]() CH3COO- + H + 10-4 mol��L 10-11 mol��L B �� �� �ڣ��ߣ��ݣ��٣��ܣ��ޣ��� 1 :9
CH3COO- + H + 10-4 mol��L 10-11 mol��L B �� �� �ڣ��ߣ��ݣ��٣��ܣ��ޣ��� 1 :9
��������
ǿ���ǿ����ȫ���룬���������ֵ��룻FeCl3����ǿ�������Σ�ˮ�������ԣ�CH3COONa����ǿ�������Σ�ˮ���Լ��ԣ�ˮ�ĵ��������ģ�����������ȣ��ɴ˷������
(1)FeCl3����ǿ�������Σ���ˮ��Һ�з���ˮ�ⷴӦ��Fe3++3H2O![]() Fe(OH)3+3H+��ʹ��Һ��c(H+)>c(OH-)����Һ�����ԡ�CH3COOH�������ᣬ��Һ�����ԣ����뷽��ʽΪ��CH3COOH
Fe(OH)3+3H+��ʹ��Һ��c(H+)>c(OH-)����Һ�����ԡ�CH3COOH�������ᣬ��Һ�����ԣ����뷽��ʽΪ��CH3COOH![]() CH3COO-+H+��
CH3COO-+H+��
(2)CH3COONa����ǿ�������Σ�ˮ��ʹ��Һ�Լ��ԣ�CH3COO-+H2O![]() CH3COOH+OH-������Һ��pH=10֪����Һ��c(H+)��Һ=10-10mol/L�����Ը���Һ����ˮ���������c(OH-)ˮ=c(OH-)��Һ=
CH3COOH+OH-������Һ��pH=10֪����Һ��c(H+)��Һ=10-10mol/L�����Ը���Һ����ˮ���������c(OH-)ˮ=c(OH-)��Һ=![]() =
=![]() =10-4mol/L����pH=3��HCl��Һ֪����HCl��Һ��c(H+)��Һ=10-3mol/L��HCl��Һ����ˮ���������c(H+)ˮ=c(OH-)��Һ=
=10-4mol/L����pH=3��HCl��Һ֪����HCl��Һ��c(H+)��Һ=10-3mol/L��HCl��Һ����ˮ���������c(H+)ˮ=c(OH-)��Һ=![]() =
=![]() =10-11mol/L��
=10-11mol/L��
(3)A.NaHSO4��ˮ�е���NaHSO4=Na++H++SO42-����������Һ��c(H+)��ˮ�ĵ���ƽ�������ƶ���A�����B.(NH4)2SO4��ˮ���ܷ���ˮ�ⷴӦ��NH4++H2O![]() NH3��H2O+H+��ʹˮ�ĵ���ƽ�������ƶ�����������Һ�����ԣ�B����ȷ��C.��ˮ�ĵ��������ȹ��̣�������ʹˮ�ĵ���ƽ�������ƶ�������Һ��c(H+)=c(OH-)��ˮ��Ȼ�����ԣ�C�����D.Na2CO3��ˮ���ܷ���ˮ�ⷴӦ��CO32-+H2O
NH3��H2O+H+��ʹˮ�ĵ���ƽ�������ƶ�����������Һ�����ԣ�B����ȷ��C.��ˮ�ĵ��������ȹ��̣�������ʹˮ�ĵ���ƽ�������ƶ�������Һ��c(H+)=c(OH-)��ˮ��Ȼ�����ԣ�C�����D.Na2CO3��ˮ���ܷ���ˮ�ⷴӦ��CO32-+H2O![]() HCO3-+OH-��ʹˮ�ĵ���ƽ�������ƶ�����������Һ�Լ��ԣ�D�����ѡB��
HCO3-+OH-��ʹˮ�ĵ���ƽ�������ƶ�����������Һ�Լ��ԣ�D�����ѡB��
(4) ��Ũ�ȵ�NaOH��Һ��NH3H2O��Һ��ϡ����ͬ�ı���ʱ����Һ��c(OH-)��С��ϵ�ǣ�NaOH>NH3H2O����ʹϡ�ͺ���ҺpH���[��c(OH-)���]����Ӧ��NaOH��Һ����ϡ�ͣ����ſ�����NH3H2O��pH��ȣ�������ϡ�ͺ�����Һ��pH��ȣ���m>n��
(5)pH=a��������c(H+)=10-amol/L��������ǿ�ᣬ�������ʵ���Ũ��Ϊ10-amol/L��pH=14-a�İ�ˮ��c(OH-)=![]() =
=![]() =10-amol/L������ˮ�������ˮ���ʵ���Ũ��Զ����10-amol/L�����кͷ�Ӧ����ʽHCl+NH3H2O=NH4Cl+H2O����֪����Ϻ�����Һ�����ԣ����������ҪԶ���ڰ�ˮ�������Va>Vb��
=10-amol/L������ˮ�������ˮ���ʵ���Ũ��Զ����10-amol/L�����кͷ�Ӧ����ʽHCl+NH3H2O=NH4Cl+H2O����֪����Ϻ�����Һ�����ԣ����������ҪԶ���ڰ�ˮ�������Va>Vb��
(6)HCl��FeCl3��CH3COOH��Һ�����ԣ�pHС��7��NaCl�����ԣ�pH����7��NaOH��CH3COONa��NH3H2O��Һ�Լ��ԣ�pH����7��һ����˵������ˮ��̶�С������ĵ���̶ȣ���Ũ��FeCl3����������HCl��CH3COOH��CH3COONa�ļ�������NaOH��NH3H2O��ǿ���ǿ����ȫ���룬���������ֵ��룬���Ե�Ũ��ʱHCl������ǿ��CH3COOH��NaOH�ļ���ǿ��NH3H2O���ܶ���֮����Ũ��ʱ����������Һ��pH�ɴ�С��˳��Ϊ��NaOH>NH3H2O>CH3COONa>NaCl>FeCl3>CH3COOH>HCl������>��>��>��>��>��>�ۡ�
(7)pH=13��NaOH��Һ��c(OH-)=![]() =
=![]() =0.1mol/L��pH=2��������Һ��c(H+)=10-2mol/L����Ϊ������ҺpH=11��˵��NaOH��������c(OH-)���=
=0.1mol/L��pH=2��������Һ��c(H+)=10-2mol/L����Ϊ������ҺpH=11��˵��NaOH��������c(OH-)���=![]() =
=![]() =10-3mol/L����c(OH-)���=
=10-3mol/L����c(OH-)���=![]() �� ����������
�� ����������![]() =10-3mol/L�����VNaOH(aq):VHCl(aq)=1:9��
=10-3mol/L�����VNaOH(aq):VHCl(aq)=1:9��


 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з���ʽ��ȷ����
A. ��KIO3����������Һ�е�KI�� 5I��+IO3��+3H2O =3I2+6OH��
B. ��ϡ����ϴ���Թ��ڱڵ������� Ag+2H��+NO3��=Ag��+NO��+H2O
C. ������SO2ͨ���䰱ˮ�У� SO2+NH3��H2O =HSO3��+NH4+
D. ��NH4HCO3��Һ�мӹ�����NaOH��Һ�����ȣ� NH4+ +OH��![]() NH3��+H2O
NH3��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӱ��ˮ�ĵ���ƽ�⣬��ʹ��Һ��c(H+)��c(OH-)�Ĵ�ʩ�ǣ� ��
A. ��ˮ������У����pH=6 B. ��ˮ�м���FeCl3����
C. ��ˮ��Ͷ��һС������� D. ��ˮ�м���Na2CO3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͭ����ᾧˮ�����ⶨ��ʵ���У������ʵ����ƫ�͵��ǣ� ��
A. ����ʱ����������岿�ֱ��B. �����ڸ��в��ӷ�����
C. ���岻�������в��ӷ�����D. ���ȹ��������������彦��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)Ϊ����֤ Fe2+�� Cu2+������ǿ��������װ���ܴﵽʵ��Ŀ�ĵ���_______����װ����ţ��� �������ĵ缫��ӦʽΪ_______����������ԭ���ʱ�����缫��������ȣ���������ͨ�� 0.4 mol ����ʱ�������缫��������Ϊ_______g��

(2)�� CH4 ��Ƴ�ȼ�ϵ�أ��������ʸ��ߣ�װ����ͼ��ʾ��A��B Ϊ���̼������
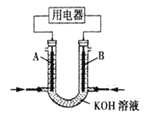
ʵ���� OH- �������� A �缫����_______���� A �� B�����缫���ͨ CH4����缫��ӦʽΪ_______��
(3)����ұ���ʹ������漰������ԭ��Ӧ������������ұ����Ӧ����ʱ���õ�ⷨ����_______����ѡ ����ĸ����
a��Fe2O3 b��NaCl c��Cu2S d��Al2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ʹ1.6 g Fe2O3��ȫ����ԭ������Ӧ����CO����ı�״���µ����Ϊ(����)
A. 672 mLB. 336 mLC. ����672 mLD. ��336 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������Ǵ�ͳ�в�ҩ���˵���Ч�ɷ�֮һ��������ϸ����ɱ���ж������á������йغ������ص�������ȷ����( )

A. �������صķ���ʽΪC16H13O5
B. ��������FeCl3��Һ��ɫ
C. 1 mol����������ˮ��Ӧ���������1 mol Br2
D. ������H2�����ӳɷ�Ӧ,�������H2 6mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��������������������Ҫ�����á������й�˵������ȷ����( )
A. ���ӽ���ʱ����![]() ��Һ�������
��Һ�������
B. �϶�ϵ��������ʹ�õ�̼��ά����һ���������ǽ�������
C. ֻҪ������������ʳ��ɫ������������������������ΪijЩʳƷ�����Ӽ�
D. PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ�����Ƿ���������������Ҫԭ����Щ��������ɢ�ڿ����ж����γɽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һƿ�������Һ�����п��ܺ���NH![]() ��K����Mg2����Ba2����Al3����Fe3����SO42-��CO32-��NO3-��I����Cl����ȡ����Һ��������ʵ�飺
��K����Mg2����Ba2����Al3����Fe3����SO42-��CO32-��NO3-��I����Cl����ȡ����Һ��������ʵ�飺
����pH��ֽ��ø���Һ�����ԣ�
��ȡ������Һ�������������Ƶ���ˮ������CCl4���������ú�CCl4����Ϻ�ɫ��
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������Ϊ���ԣ��������μӹ������������ɣ�
��ȡ��������������Һ������Na2CO3��Һ���а�ɫ�������ɣ�
�ݽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵ�ش����⣺
��1��д������������Ӧ�����ӷ���ʽ_________��
��2������Һ�п϶����ڵ�������________��
��3������Һ�п϶������ڵ�������________��
��4������Һ�л�����ȷ���Ƿ���ڵ�������_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com