��������Ҫ�ɰ�������ɣ�ƽ��������������������Ϊ16%���ң�����ͨ�еļ�⵰���ʵķ����ǡ���ʽ��������--�Բ���ĺ���������һ��ϵ�������㵰���ʵĺ���������ǰ������ijЩ���ϸ��̷ۺ�α���̷��е����ʺ�������꣬�����˰��ո�������ͷ���ޡ��¼��ķ����������ع���������ꡰ��ʽ���������ļ��©����ͨ��������Ʒ�����Ӻ������ߴ�66.7%�������谷��C
3H
6N
6������ɵ����ʺ������ļ����ɴ��ֵ�����ʯ��ׯ��¹�������̷ۡ��¼��ķ�����
��1���������ʵĺ�������16%�ƣ���ʽ�����������Ժ����������㵰���ʺ��������Ե�ϵ��ӦΪ
��
��2����ʽ����������Ҫԭ��Ϊ������Ʒ������ʹ���һ����ȣ�ʹ�����ʷֽ⣬�����İ����������պ������������Һ�ζ�������������������Ի���ϵ������Ϊ�����ʺ�����ij����С��ͬѧ�ⶨҺ̬�̺�������ʵ��������£�
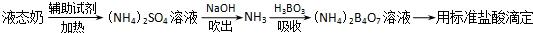
����岽�����£�
�����ձ��м���10.00mLҺ̬�̺����Լ������ȳ�ַ�Ӧ��
����ӦҺת�Ƶ����Թ��У�
����ͼװ������Թ��м���10.00mL 40%��NaOH��Һ������ˮ���������ɵ�NH
3����������H
3BO
3��Һ���մ�����NH
3������װ��δ��������
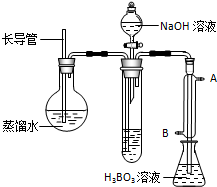
����ȡ����ƿ���μ�ָʾ������0.1000mol/L�����Һ�ζ���
�����ظ��ⶨ���Σ�����10.00mL����ˮ����Һ̬�̽�������������
�й����ݼ�¼���£�
| ��� |
��Ʒ�����Լ� |
����������� |
| 1 |
10.00mLҺ̬�̡�0.2g������20mLŨ���� |
33.45mL |
| 2 |
10.00mLҺ̬�̡�0.2g������20mLŨ���� |
33.55mL |
| 3 |
10.00mLҺ̬�̡�0.2g������20mLŨ���� |
33.50mL |
| 4 |
10.00mL����ˮ��0.2g������20mLŨ���� |
1.50mL |
��ش��������⣺
���ڲ�����ʵ��װ���У���Ҫ���ȵ�������
�����������ƣ��������ܵ�������
�����Թ�����������Ӧ�����ӷ���ʽ��
����ȴˮӦ��
������ĸ���ţ��ڽ��룮
�����4�ſհ���ʵ���Ŀ����
��
�۵ζ�ʱ��NH
4��
2B
4O
7����ת��ΪH
3BO
3����Ӧ�Ļ�ѧ����ʽΪ
��
�ܼ���10.00mLҺ̬���еĺ�����Ӧ�������ʽ�����������
mL����Һ̬�̵ĺ�����Ϊ
mg/mL��

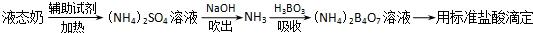



 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�