
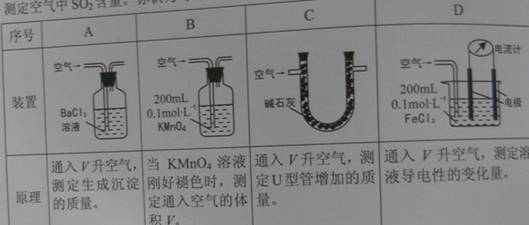
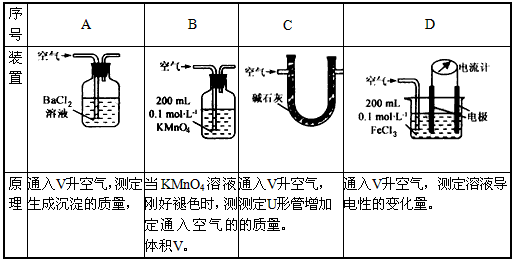
��.��֪��Һ����������������Ũ���ж�����ϵ��ij��ȤС�����������װ�úͷ����ⶨ������SO2����������Ϊ���еIJ�����________________(�����)
��.Fe2+��SO32-��I-�����л�ԭ�ԡ�Ϊ�˱Ƚ��仹ԭ��ǿ������������̽�����ش�
��1����֪���ٻ�ԭ�ԣ�SO32-> Fe2+����FeSO3������ˮ��
��2���ڣ�1���Ļ����ϣ�������м��衣
����1����ԭ�ԣ�I->SO32-> Fe2+��
����2����ԭ�ԣ� SO32-> I- >Fe2+��
����2����ԭ�ԣ� ______________________��
(3)ʵ��̽�����ֶԼ���2����̽�������ʵ�鲽���Լ�Ԥ�ڵ�����ͽ��ۡ�
��ѡ�����Լ���0.1mol/L Na2SO3��0.1mol/L KI��0.1mol/L FeCl3��10%KSCN�����Ʊ�����ˮ��������Һ��ϡHNO3��1mol/L HCl��1mol/L BaCl2
|
|
Ԥ�ڵ������� |
|
����1��ȡ1mL0.1mol/L Na2SO3��1mL0.1mol/L KI��Һ���Թ��У���Ϻ����2~3��������ˮ���� |
|
|
����2��____________________________ _____________________________________ |
____________________________________ _______________________________________ |
|
|
|
|
����4��____________________________ _____________________________________ |
____________________________________ _______________________________________ |
��BD
����3����ԭ�� SO32����Fe2+��I��
ѧ������ʱ�����ܳ��ֵļ������Σ����ܰ�����ˮ��������Ƽ�������
|
ʵ����� |
Ԥ����������� |
|
����2�� ���Թ��м��������� 1 mol��L-1HCl ���ٵ������Σ������� 1 mol��L-1BaCl2�����Թ� |
��û�а�ɫ������������˵����ԭ�ԣ�I����SO32�� ����û�а�ɫ������������˵������2������ ����û�а�ɫ������������˵������1���� |
|
����2��������2�����Թ��е�������������Һ�����Թ� |
��������ɫ��˵����ԭ�ԣ�SO32����I�� ��������ɫ��˵����ԭ�ԣ�I����SO32�� |
|
����4��������1�����Թ��е�������������Һ �����Թ� |
��������ɫ��˵����ԭ�ԣ�Fe2+��I�� |
|
����4��������2�����Թ��е�������10% KSCN��Һ�����Թ� |
�������ɫ��˵����ԭ�ԣ� �����ɫ��˵����ԭ�ԣ�Fe2+��I�� |
����������


 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д� ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.��֪��Һ����������������Ũ���ж�����ϵ��ij��ȤС�����������װ�úͷ����ⶨ������SO2����������Ϊ���еIJ�����________________(�����)

��.Fe2+��SO32-��I-�����л�ԭ�ԡ�Ϊ�˱Ƚ��仹ԭ��ǿ������������̽�����ش�
��1����֪���ٻ�ԭ�ԣ�SO32-> Fe2+����FeSO3������ˮ��
��2���ڣ�1���Ļ����ϣ�������м��衣
����1����ԭ�ԣ�I->SO32-> Fe2+��
����2����ԭ�ԣ� SO32-> I- >Fe2+��
����2����ԭ�ԣ� ______________________��
(3)ʵ��̽�����ֶԼ���2����̽�������ʵ�鲽���Լ�Ԥ�ڵ�����ͽ��ۡ�
��ѡ�����Լ���0.1mol/L Na2SO3��0.1mol/L KI��0.1mol/L FeCl3��10%KSCN�����Ʊ�����ˮ��������Һ��ϡHNO3��1mol/L HCl��1mol/L BaCl2
|
| Ԥ�ڵ������� |
| ����1��ȡ1mL0.1mol/L Na2SO3��1mL0.1mol/L KI��Һ���Թ��У���Ϻ����2~3��������ˮ���� | |
| ����2��____________________________ _____________________________________ | ____________________________________ _______________________________________ |
|
| |
| ����4��____________________________ _____________________________________ | ____________________________________ _______________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.��֪��Һ����������������Ũ���ж�����ϵ��ij��ȤС�����������װ�úͷ����ⶨ������SO2����������Ϊ���еIJ�����________________(�����)
��.Fe2+��SO32-��I-�����л�ԭ�ԡ�Ϊ�˱Ƚ��仹ԭ��ǿ������������̽�����ش�
��1����֪���ٻ�ԭ�ԣ�SO32-> Fe2+����FeSO3������ˮ��
��2���ڣ�1���Ļ����ϣ�������м��衣
����1����ԭ�ԣ�I->SO32->Fe2+��
����2����ԭ�ԣ� SO32->I- >Fe2+��
����2����ԭ�ԣ� ______________________��
(3)ʵ��̽�����ֶԼ���2����̽�������ʵ�鲽���Լ�Ԥ�ڵ�����ͽ��ۡ�
��ѡ�����Լ���0.1mol/L Na2SO3��0.1mol/L KI��0.1mol/L FeCl3��10%KSCN�����Ʊ�����ˮ��������Һ��ϡHNO3��1mol/L HCl��1mol/L BaCl2
|
| Ԥ�ڵ������� |
| ����1��ȡ1mL0.1mol/L Na2SO3��1mL0.1mol/L KI��Һ���Թ��У���Ϻ����2~3��������ˮ���� |
|
| ����2��____________________________ _____________________________________ | ____________________________________ _______________________________________ |
|
|
|
| ����4��____________________________ _____________________________________ | ____________________________________ _______________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ�����и�����һ�ε��п��ԣ����ۣ���ѧ���� ���ͣ������
��.��֪��Һ����������������Ũ���ж�����ϵ��ij��ȤС�����������װ�úͷ����ⶨ������SO2����������Ϊ���еIJ�����________________(�����)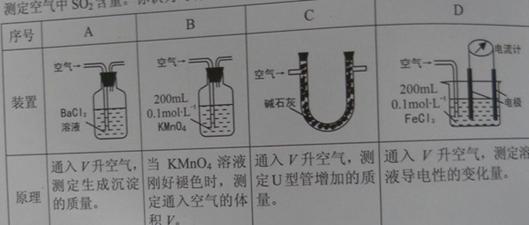
��.Fe2+��SO32-��I-�����л�ԭ�ԡ�Ϊ�˱Ƚ��仹ԭ��ǿ������������̽�����ش�
��1����֪���ٻ�ԭ�ԣ�SO32-> Fe2+����FeSO3������ˮ��
��2���ڣ�1���Ļ����ϣ�������м��衣
����1����ԭ�ԣ�I->SO32-> Fe2+��
����2����ԭ�ԣ� SO32-> I- >Fe2+��
����2����ԭ�ԣ� ______________________��
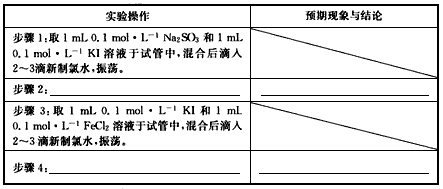
(3)ʵ��̽�����ֶԼ���2����̽�������ʵ�鲽���Լ�Ԥ�ڵ�����ͽ��ۡ�
��ѡ�����Լ���0.1mol/L Na2SO3��0.1mol/L KI��0.1mol/L FeCl3��10%KSCN�����Ʊ�����ˮ��������Һ��ϡHNO3��1mol/L HCl��1mol/L BaCl2
 ʵ����� ʵ����� | Ԥ�ڵ������� |
| ����1��ȡ1mL0.1mol/L Na2SO3��1mL0.1mol/L KI��Һ���Թ��У���Ϻ����2~3��������ˮ���� | |
| ����2��____________________________ _____________________________________ | ____________________________________ _______________________________________ |
 ����3��ȡ1mL0.1mol/L KI��1mL0.1mol/LFeCl2��Һ���Թ��У���Ϻ����2~3��������ˮ���� ����3��ȡ1mL0.1mol/L KI��1mL0.1mol/LFeCl2��Һ���Թ��У���Ϻ����2~3��������ˮ���� | |
| ����4��____________________________ _____________________________________ | ____________________________________ _______________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡģ���� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com