
����Ŀ����ѧ�Ľ����벻��������ͻ�ơ�ԭ�ӹ��˴Ź���X�������䡢���Ӽ���ȼ����ķ�չ��Ӧ�ö��ƽ��˽ṹ���о��������Ԫ��ԭ�ӽṹ�������ӽṹ������ṹ�������ṹ�о��ȡ�
(1)����Ԫ��Crԭ�ӵĻ�̬�����Ų�ʽΪ______��Cr�ĺ�������ɼ���̬ԾǨ����̬ʱ�����Ĺ�����______ (����ա����䡱)���ס�
(2)���f����[C(NH2)3+]������������(CH3SO3-)�γɳ����Ӿ��壬��ֲ��ṹ��ͼ��ʾ��

����ɸþ����Ԫ���е縺��������______������S���ӻ�������_______��
��Ԫ��C��N��S�ļ��⻯����ˮ�е��ܽ�ȴ�С�����˳��Ϊ______��ԭ����______��
(3)�ٽ�����ͨ��CuSO4��Һ�У�������ɫ����������ͨ���������������ܽ⣬�õ���ɫ����Һ���ù��������ı仯��[Cu(H2O)4]2+��Cu(OH)2��[Cu(NH3)4]2+��[Cu(H2O)4]2+��[Cu(NH3)4]2+�й�ͬ���еĻ�ѧ��������_________��
����֪:AlF63+����Һ�п��ȶ����ڣ�CaF2������ˮ���������ں�Al3+����Һ�У�ԭ����_____(�����ӷ���ʽ��ʾ)��
(4)�����о���Աͨ�����ӻ�ѧ����Ԥ�Ⲣ�ϳ��˻�����Na2He����X������������侧���ṹ��ͼ��ʾ��

�پ�����Na�ѻ��γ�___________(����״)�ռ�ṹ��Heռ��һ���϶����һ����e-ռ�ݡ���֪Na2He���岻�ܵ��磬������__________��
����֪�����߳�Ϊanm��������Na�İ뾶Ϊbnm����He�İ뾶Ϊ_____nm(�г�����ʽ����)��
���𰸡� [Ar]3d54s1(��1s22s22p63s23p63d54s1) ���� O sp3 CH4 <H2S< NH3 CH4Ϊ�Ǽ��Է��ӣ�NH3��H2S��Ϊ���Է��ӣ���NH3����ˮ�γ���� ���Լ�����λ�� 3CaF2+Al3+=3Ca2++AlF63- ������ û�������ƶ������Ӻ͵��� ![]()
����������1������Ԫ��Crԭ�ӵĻ�̬�����Ų�ʽΪ��[Ar]3d54s1(��1s22s22p63s23p63d54s1)��Cr�ĺ�������ɼ���̬ԾǨ����̬ʱ�����Ĺ����Ƿ�����ף��ʴ�Ϊ��[Ar]3d54s1(��1s22s22p63s23p63d54s1)�����䣻
��2���ٵ縺������Ԫ��ΪOԪ�أ�S�γ���4���Ҽ������ݼ۲���ӶԻ���ģ���ж�SΪsp3�ӻ���
��ˮΪ�����ܼ���CH4Ϊ�Ǽ��Է��ӣ�NH3��H2S��Ϊ���Է��ӣ���NH3����ˮ�γ�������ٽ����������ԭ�������ж�C��N��S�ļ��⻯����ˮ�е��ܽ�ȴ�С����Ϊ��CH4 <H2S< NH3���ʴ�Ϊ��CH4 <H2S< NH3��CH4Ϊ�Ǽ��Է��ӣ�NH3��H2S��Ϊ���Է��ӣ���NH3����ˮ�γ������
��3���� ˮ���ӻ��������ж����ڼ��Լ�,Cuԭ�Ӻ�ˮ���ӻ�������֮�������λ��,����[Cu(H2O)6]2+��[Cu(NH3)4]2+�ж�������λ�������Լ����ʴ�Ϊ����λ���ͼ��Լ���
��CaF2������ˮ���������ں�Al3+����Һ�У���Ϊ����Һ��F-��Al3+���γɺ��ѵ����������AlF63��ʹCaF2���ܽ�ƽ�����ƣ��䷴Ӧ�����ӷ���ʽΪ��3CaF2+Al3+=3Ca2++AlF63-��
��4���پ�������ͼ�кڵ���Na��8��Na�γɵ�����ṹ�������壬Na2He��û�������ƶ������Ӻ͵��ӣ����Բ��ܵ��磬�ʴ�Ϊ�������壻û�������ƶ������Ӻ͵��ӡ�
���ɾ����Ľṹ���Է�����֪��Na��He֮��ľ���Ϊ������Խ��ߵ�1/4����Խ��߳�![]() ��Na��Heֱ�ӽӴ�����He�İ뾶Ϊ
��Na��Heֱ�ӽӴ�����He�İ뾶Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڵ����Ȳ��ĵ��Ӿ硶��������塷�У��������ڲμ�ʡί�ٿ��ġ���չ�������࣬�����������š�������顣�����ʻ����ֳ�: ������һ���������ǿɻ�������
A. ��Ƥ���ɱ�ֽ���ϵ�� B. ʣ�������ͷ������ƿ
C. ����ƿ��������ƿ������ҵ�� D. �����ޡ�������ֻ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ��������,ͨ�����з�Ӧ�����Ʊ������մɵ�ԭ��Mg0:MgSO4(s)+CO(g) ![]() MgO(s)+CO2(g)+SO2(g)��H>0 �÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������
MgO(s)+CO2(g)+SO2(g)��H>0 �÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������

ѡ�� | x | y |
A | ѹǿ | CO2��CO�����ʵ���֮�� |
B | �¶� | �����ڻ��������ܶ� |
C | MgSO4������(�������) | CO��ת���� |
D | SO2��Ũ�� | ƽ�ⳣ��K |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ѳ�����ɫ��������Ӳ��ǿ�ȴ����ȡ��ܶ�С������Ϊ����������Ŀǰ�����Ѳ����Ȼ������������ʯ���������뽹̿��ϣ�ͨ�������������Ƶ�TiCl4��2FeTiO3+7Cl2+6C![]() 2TiCl4+2FeCl3+6CO�� TiO2+2Cl2+2C
2TiCl4+2FeCl3+6CO�� TiO2+2Cl2+2C![]() TiCl4+2CO����TiCl4�����ᴿ���������������þ���ȵõ��ѣ�TiCl4+2Mg
TiCl4+2CO����TiCl4�����ᴿ���������������þ���ȵõ��ѣ�TiCl4+2Mg![]() Ti+2MgCl2��MgCl2����Mg��ϡ�����ܽ��ú���״�ѣ���������ۻ������Ѷ�����ش��������⣺
Ti+2MgCl2��MgCl2����Mg��ϡ�����ܽ��ú���״�ѣ���������ۻ������Ѷ�����ش��������⣺
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ_______________________________��
��2����CO��Ϊ�ȵ����������Ϊ_____���ѧʽ����
��3����CH2Cl2��C6H6��CO2��C2H4�У�̼ԭ�Ӳ�ȡsp�ӻ��ķ�����__________ ��
��4��TiCl4�ڳ���������ɫҺ�壬��ˮ��ʪ��������ˮ���ð���̡���TiCl4����______(�ԭ�ӡ��������ӡ������ӡ�)���塣
��5������ͬ���ڵ���һ��Ԫ����(Co)���γɷ���ʽ��ΪCo(NH3)5BrSO4��������������һ�ֻ�ѧʽΪ[Co(NH3)5Br]SO4��������Һ�м�BaCl2��Һʱ��������_____________������һ����������Һ�м���BaCl2��Һʱ������������������AgNO3��Һʱ����������ɫ��������ڶ��������Ļ�ѧʽΪ __________��
��6������Ȼ����TiO2�н��ʯ�����ѿ����ѿ����־��ͣ����н��ʯ�ľ�������ͼ��ʾ��������Ti4+����λ��Ϊ__________��
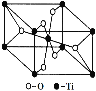
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Se)�����彡�������һ����Ԫ�أ���֪Se��ԭ�ӽṹʾ��ͼ��ͼ������˵������ȷ����

A. ��ԭ�ӵ�������Ϊ34
B. ��Ԫ�ش��ڵ������ڵڢ�A��
C. SeO2�������������л�ԭ��
D. ���ԣ�H2SO4>H2SeO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����20g���ֽ����Ļ����Ͷ��������ϡ�����У���Ӧ��ȫ��õ���״����H2 11.2L����û�������ɿ�����
A. Mg��Cu B. Na��Fe C. Al��Mg D. Zn��Fe
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҳ�������װ��������ͭ��Ũ���ᷴӦ��һϵ��ʵ�顣
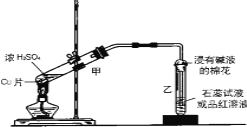
(1)д����װ���з�������Ҫ��Ӧ�Ļ�ѧ����ʽ____________________________��
(2)װ���ҵ��Թܿڲ�����һ�Ž��м�Һ����������ͨ���ǽ��б���̼������Һ����������_________________________��
(3)���ɵ�����ʹƷ����Һ����ɫ______________��ʵ����Ϻ�ȡ�������Թ�����Һ���Թ��м��ȣ�������________________��ԭ����__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����У��й��Լ��ı��淽��������ǣ� ��
A. �ռ���Һ�����ڴ����������Լ�ƿ��B. Һ����ˮ�Ᵽ��
C. ���������Ʊ�����ú����D. Ũ���ᱣ������ɫϸ��ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʡ�����֮�������·�Ӧ��ϵ��
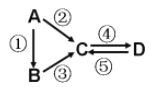
��1����B�ǵ���ɫ���壬��Ӧ�ڢ۾��õ�ͬһ��Һ̬�⻯�D���ʳ�����ʳƷ��ҵ��д����Ӧ�ܵĻ�ѧ����ʽ��__________________________________________��
��2����B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��д����Ӧ�۵Ļ�ѧ����ʽ��_____________________________________________________��
��3����A��Ӧ����㷺�Ľ�������Ӧ���õ�A����Ӧ�ڢݾ��õ�ͬһ�ַǽ������ʡ�д����Ӧ�ܵ����ӷ���ʽ��_________________________________________________________��
��4����D���ʾ������ԣ���Ӧ�ڢ۾�Ҫ��ǿ����Һ����Ӧ����ͨ�������һ����������ЧӦ����Ҫ���塣д����Ӧ�ܵ����ӷ���ʽ��_____________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com