
| ���� |
| �� |
| HNO3(ϡ) |


 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�����а��и����������¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(12��)���ᡢ��������ᶼ����Ҫ�Ļ���ԭ�ϣ�Ҳ�ǻ�ѧʵ������ر�����Ҫ�Լ�����ش��������⣺
��1�������£������������Ƶ�����ʢ��Ũ���ᣬ˵��Ũ������� �ԡ��ò�����պȡŨ�������ֽ�ϣ�ֽ��ڣ�˵��Ũ�������  �ԡ�
�ԡ�
��2������100mL 18mol��L��1��Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ�������������ڱ�״���µ���������� ��
| A��40.32L | B��30.24L | C��20.16L | D��13.44L |
 Ӧ�Ļ�ѧ����ʽΪ ��
Ӧ�Ļ�ѧ����ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�������и��������ν���������⻯ѧ�Ծ����������� ���ͣ������
(14��)���ᡢ��������ᶼ����Ҫ�Ļ���ԭ�ϣ�Ҳ�ǻ�ѧʵ������ر�����Ҫ�Լ�����ش��������⣺
��1�������£������������Ƶ�����ʢ��Ũ���ᣬ˵��Ũ������� �ԡ��ò�����պȡŨ�������ֽ�ϣ�ֽ��ڣ�˵��Ũ������� �ԡ�
��2������ͭ���Ʊ�Cu-Zn-Alϵ��������Ҫԭ�ϣ���ҵ����ϴ���ķ�ͭм��ԭ�����Ʊ�����ͭ�������Ʊ��������ϡ���ɫ��ѧ��˼����� ������ţ���
�� Cu + HNO3��Ũ���� Cu(NO3)2 �� Cu + HNO3��ϡ���� Cu(NO3)2
�� Cu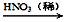 CuO
CuO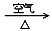 Cu(NO3)2
Cu(NO3)2
��3������100mL 18mol��L-1��Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����ò����������ڱ�״���µ���������� ��
| A��40.32L | B��30.24L | C��20.16L | D��13.44L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���㽭ʡ������ѧ�ڵ�����ͳ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
���ᡢ��������ᶼ����Ҫ�Ļ���ԭ�ϣ�Ҳ�ǻ�ѧʵ������ر�����Ҫ�Լ�����ش��������⣺

��1�� �����£������������Ƶ�����ʢ��Ũ���ᣬ˵��Ũ������� �ԣ��ò�����պȡŨ�������ֽ�ϣ�ֽ��ڣ�˵��Ũ������� �ԣ�ʵ���Ҳ�����Ũ������ﰱ����˵��������� �ԡ�
��2�� ����ͭ���Ʊ�Cu-Zn-Alϵ��������Ҫԭ�ϣ���ҵ����ϴ���ķ�ͭм��ԭ�����Ʊ�����ͭ����ͼ��ʾ�Ʊ��������ϡ���ɫ��ѧ��˼���
�� (�����)��
��3�� ����100ml 18 mol��LŨ�����м������ͭƬ������ʹ֮��ַ�Ӧ�������������ڱ�״���µ���������� ��
A��40.32 L B��30.24 L C��20.16 L D��13.44 L
����ʹ������Ӧ��ʣ���ͭƬ�����ܽ⣬�������м��������ƣ�д����Ӧ�����ӷ���ʽ ��
��4�� ijͬѧ�����ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬���Ҹ÷�Ӧ�IJ���ֻ���Ȼ�ͭ��ˮ����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�人����У����10��������ѧ�Ծ��������棩 ���ͣ������
(10��)���ᡢ��������ᶼ����Ҫ�Ļ���ԭ�ϣ�Ҳ�ǻ�ѧʵ������ر�����Ҫ�Լ�����ش��������⣺
��1�������£������������Ƶ�����ʢ��Ũ���ᣬ˵��Ũ������� �ԡ��ò�����պȡŨ�������ֽ�ϣ�ֽ��ڣ�˵��Ũ������� �ԡ�
��2������ͭ���Ʊ�Cu-Zn-Alϵ��������Ҫԭ�ϣ���ҵ����ϴ���ķ�ͭм��ԭ�����Ʊ�����ͭ�������Ʊ��������ϡ���ɫ��ѧ��˼����� ������ţ���
�� Cu + HNO3��Ũ���� Cu(NO3)2 �� Cu + HNO3��ϡ���� Cu(NO3)2
�� Cu 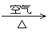 CuO
CuO
 Cu(NO3)2
Cu(NO3)2
��3������100mL 18mol��L-1��Ũ�����м��������ͭƬ������ʹ֮��ַ�Ӧ����ò����������ڱ�״���µ���������� ��
A��40.32L B��30.24L C��20.16L D��13.44L
����ʹ������Ӧ����ʣ���ͭƬ�����ܽ⣬�������м��������ƣ�д����Ӧ�����ӷ���ʽ ��
��4��ijͬѧ�����ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬���Ҹ÷�Ӧ�IJ���ֻ���Ȼ�ͭ��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com