
��10�֣���1�������£���20.0g14%��NaCl��Һ��30.0g24%��NaCl��Һ��ϣ���Ϻ�õ��ܶ�Ϊ1.17g/cm3����Һ������㣺��������̲��÷֣�
�ٻ�Ϻ���Һ��NaCl����������
�ڻ�Ϻ���Һ��NaCl�����ʵ���Ũ��
����1000gˮ�мӶ��� mol NaCl������ʹ��Ũ��ǡ����������Ϻ���Һ��Ũ����ȡ����������һλС����
��2����״���£�11.2LO2��CO2������������Ϊ19.6g������������O2��CO2�����֮��
��1���ٻ�Ϲ����������Dz����
���Ի�Ϻ���Һ��NaCl������������
�ڸ��� ��֪��Ϻ���Һ��NaCl�����ʵ���Ũ����
��֪��Ϻ���Һ��NaCl�����ʵ���Ũ����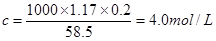
�۸�������������֪ �����x��4.3mol
�����x��4.3mol
��2������������O2��CO2�����ʵ����ֱ���x��y
��״���£�11.2L����������ʵ�����11.2L��22.4L/mol��0.5mol
������x��y��0.5mol��32x��44y��19.6g
���x��0.2mol��y��0.3mol
���Ի��������O2��CO2�����֮����2:3
����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�������£���20.0 g 14%��NaCl��Һ��30.0 g 24%��NaCl��Һ��ϣ��õ��ܶ�Ϊ1.17 g/cm3�Ļ����Һ,�û����Һ��NaCl�����ʵ���Ũ��Ϊ mol/L��
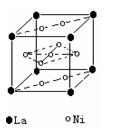
��2�����������������Դ���磨La��������Ni���ĺϽ����������ϡ���ͼΪ�úϽ�ľ���ṹ����С���ظ��ṹ��Ԫ����������һ����ԭ�ӣ�������ԭ�Ӷ������ϣ���ԭ�Ӷ��ڶ����ϡ��þ���Ļ�ѧʽΪ ��
��3��������ˮ����ˮ����Ի�����ɵ���ȾԽ��Խ���أ�����ר����Ϊ�����ý���þ��ˮ���е�NO![]() ��ԭΪN2���Ӷ�������Ⱦ��
��ԭΪN2���Ӷ�������Ⱦ��
��д��þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ ��
��������Ӧ�У����ɱ�״����33��6L����ʱ��ת�Ƶ��ӵ����ʵ���Ϊ mol������֪����þ���ԴӺ�ˮ����ȡ��MgCI2ͨ������Ƶõģ���Ҫ��ȥ����Ԫ��0.3mol�ķ�ˮ�е�NO![]() ����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg��
����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�������£���20.0 g 14%��NaCl��Һ��30.0 g 24%��NaCl��Һ��ϣ��õ��ܶ�Ϊ1.17 g/cm3�Ļ����Һ,�û����Һ��NaCl�����ʵ���Ũ��Ϊ mol/L��
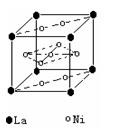
��2�����������������Դ���磨La��������Ni���ĺϽ����������ϡ���ͼΪ�úϽ�ľ���ṹ����С���ظ��ṹ��Ԫ����������һ����ԭ�ӣ�������ԭ�Ӷ������ϣ���ԭ�Ӷ��ڶ����ϡ��þ���Ļ�ѧʽΪ ��
��3��������ˮ����ˮ����Ի�����ɵ���ȾԽ��Խ���أ�����ר����Ϊ�����ý���þ��ˮ���е�NO![]() ��ԭΪN2���Ӷ�������Ⱦ��
��ԭΪN2���Ӷ�������Ⱦ��
��д��þ�ͺ�����ˮ��Ӧ�����ӷ���ʽ ��
��������Ӧ�У����ɱ�״����33��6L����ʱ��ת�Ƶ��ӵ����ʵ���Ϊ mol������֪����þ���ԴӺ�ˮ����ȡ��MgCI2ͨ������Ƶõģ���Ҫ��ȥ����Ԫ��0.3mol�ķ�ˮ�е�NO![]() ����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg��
����������Ҫ��0.5%������������MgCl2�ĺ�ˮ kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ��һ��һ���¿���ѧ�Ծ��������棩 ���ͣ�������
��10�֣���1�������£���20.0g14%��NaCl��Һ��30.0g24%��NaCl��Һ��ϣ���Ϻ�õ��ܶ�Ϊ1.17g/cm3����Һ������㣺��������̲��÷֣�
�ٻ�Ϻ���Һ��NaCl����������
�ڻ�Ϻ���Һ��NaCl�����ʵ���Ũ��
����1000gˮ�мӶ��� mol NaCl������ʹ��Ũ��ǡ����������Ϻ���Һ��Ũ����ȡ����������һλС����
��2����״���£�11.2LO2��CO2������������Ϊ19.6g������������O2��CO2�����֮��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com