

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ȷ���� ��
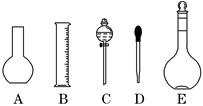
ȷ���� ��| A��ʹ����ֽ�����������ƹ��� |
B������ƿ��ԭ������������ ��ˮ ��ˮ |
| C��ת����Һ����ձ�δ�����ϴ�� |
| D����ͷ�ιܼ�ˮ����ʱ���ӿ̶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

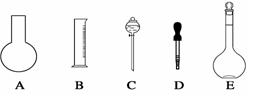
| A��װ�âٳ����ڷ��뻥�����ܵ�Һ�� | B��װ�âڿ���������NH3 |
| C��װ�âۿ������Ʊ��������� | D��װ�âܿ������ռ�NO��CO2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȡ7.68gCuSO4���壬����480mLˮ | B����ȡ12.0g�������Ƴ�480mL��Һ |
| C����ȡ8.0gCuSO4���壬����500mLˮ | D����ȡ12.5 g�������Ƴ�500mL��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
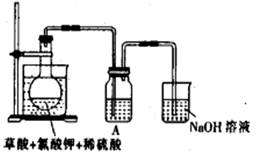
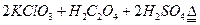
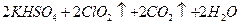
| A��20mL 60�����ˮ | B��100mL��ˮ |
| C��100mL����ʳ��ˮ | D��100mL��ˮ |
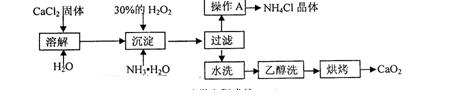
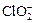 )������ˮ��ClO2��
)������ˮ��ClO2��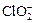 �ĺ��������������������вⶨ��ʵ�鲽�����£�
�ĺ��������������������вⶨ��ʵ�鲽�����£�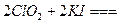
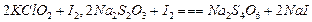 ������4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ
������4�м����ָʾ��Ϊ ���ζ��ﵽ�յ�ʱ��Һ����ɫ�仯Ϊ 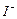 ����Һ�е�
����Һ�е�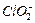 ��ԭΪ
��ԭΪ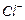 �Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��
�Բⶨ�京�����÷�Ӧ�����ӷ���ʽΪ��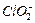 �ĺ������꣬�������м���������
�ĺ������꣬�������м���������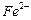 ��
��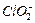 ��ԭΪ
��ԭΪ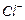 ����÷�Ӧ����������Ϊ (�ѧʽ)
����÷�Ӧ����������Ϊ (�ѧʽ)�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com