
ijϡ�����ϡ����Ļ����Һ200 mL��ƽ���ֳ����ݣ�������һ��������ͭ�ۣ�������ܽ�9.6 g������һ�����������ۣ�������������������������ӵı仯����ͼ��ʾ(��֪����ֻ����ԭΪNO����)�����з��������������

A��ԭ�������HNO3�����ʵ���Ϊ0.1 mol
B��OA�β�������NO��AB�εķ�ӦΪFe��2Fe3�� = 3Fe2����BC�β�������
C���ڶ�����Һ����������ΪFeSO4
D��H2SO4Ũ��Ϊ2.5 mol��L��1
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016�����ʡ�����и�����ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���⾫ѧ������������ޣ���ṹ��ʽ����ͼ���й����⾫��˵������ȷ����
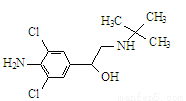
A����ѧʽΪC12H18ON2H2Cl2
B�����Է���ȡ�����ӳɡ�ˮ�⡢��������ȥ��Ӧ
C�����ڷ����廯����
D����FeCl3��Һ������ɫ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ��ɰ�һ�С�������и߶�������������ѧ�Ծ��������棩 ���ͣ�ѡ����
��Ӧ2X(g) + Y(g)  2Z(g)������Ӧ���ȣ����ڲ�ͬ�¶ȣ�T1. T2����ѹǿ��P1. P2���£���������ʵ�����n����ʱ��Ĺ�ϵͼ���£��������ж���ȷ����
2Z(g)������Ӧ���ȣ����ڲ�ͬ�¶ȣ�T1. T2����ѹǿ��P1. P2���£���������ʵ�����n����ʱ��Ĺ�ϵͼ���£��������ж���ȷ����

A��T1<T2 P1<P2
B��T1<T2 P1>P2
C��T1>T2 P1>P2
D��T1>T2 P1<P2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶���ѧ�����У�������ѧ�Ծ��������棩 ���ͣ�ѡ����
һ�������µķ�Ӧ��PCl5(g)  PCl3(g)��Cl2(g)����H��0���ﵽƽ����������ʹPCl5�ֽ��ʽ��͵���
PCl3(g)��Cl2(g)����H��0���ﵽƽ����������ʹPCl5�ֽ��ʽ��͵���
A���¶ȡ�������䣬������� B��������䣬����ϵ����
C���¶ȡ�������䣬�������� D���¶Ȳ��䣬�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶���ѧ�����У��ģ���ѧ�Ծ��������棩 ���ͣ�ѡ����
���������к����彡������
A��ʳ�������������� B��ţ�������������谷
C��ʳƷ������ά���� D��ţ�������������ĸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫ʡ�߶���ѧ���������ۻ�ѧ�Ծ��������棩 ���ͣ������
I���ס������ص缫���϶���������̼������ͼ������ش��������⣺
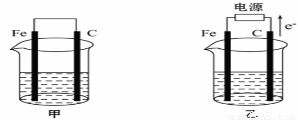
��1���������о�ʢ��CuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������ǣ��׳��е�________�����ҳ��е�________����
�����ҳ��������ĵ缫��Ӧʽ��________________________________��
��2���������о�ʢ�ű���NaCl��Һ��
��д���׳��и����ĵ缫��Ӧʽ__________________________________��
��д���ҳ��е��ܷ�Ӧ�����ӷ���ʽ______________________________��
II���ҹ�����ظ�ԭ��������Ϊ����ȼ�����Ļ���������Դ��
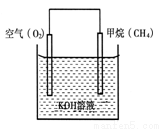
��1��CH4����Cl2��Ӧ����Ӧ��������
��Cl2��2Cl ��H = 243 kJ��mol-1
��Cl��CH4��CH3��HCl ��H = 4 kJ��mol-1
��CH3��Cl2��CH3Cl��Cl ��H = -106 kJ��mol-1
��CH4��Cl2��Ӧ����CH3Cl��g�����Ȼ�ѧ����ʽΪ __��
��2��CH4���������ȼ�ϵ�أ�����ȼ�ϵ�صĹ���ԭ����ͼ��ʾ����ͨ��CH4��һ��Ϊԭ��ص� ������������������������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫ʡ��һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������100mL1.0mol/LCuSO4��Һ����ȷ�ķ�����
A����16.0g CuSO4����100mLˮ��
B����25.0g CuSO4��5H2O��������ˮ�У�����ˮϡ����100mL
C����20mL5.0mol/L CuSO4��Һ��ˮϡ����100mL
D����20mL5.0mol/L CuSO4��Һ�м���80mLˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ��������У�߶���ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
������������ȷ����
A�������£�10mL0.02 mol•L?1 HCl��Һ��10mL0.02 mol•L?1 Ba��OH��2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20mL������Һ��pH=11
B����0.1 mol•L?1CH3COONa��Һ�У�c��OH������c��CH3COOH����c��H����
C���к����ʵ���Ũ�����������ͬ������ʹ�����Һ������NaOH�����ʵ�����ͬ
D���������Ȼ�����ҺʱӦ�����Ȼ�������Ũ���ᣬ�ټ�ˮϡ��������Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��㶫ʡ��һ��ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ������
��ˮ����Ư��������Ϊ���� ,��ˮ�Ի���ɫ����Ϊ���� .
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com