


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���и�ѡ���мס��ҵĹ�ϵ������ʾ��ͼ��ʾ���ǣ������� ���и�ѡ���мס��ҵĹ�ϵ������ʾ��ͼ��ʾ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| һ������ |
| ʱ��/min | 0 | 30 | 70 | 80 | 100 |
| n��CO2��/mol | 0.10 | 0.060 | 0.040 | 0.040 | 0.040 |
�鿴�𰸺ͽ���>>
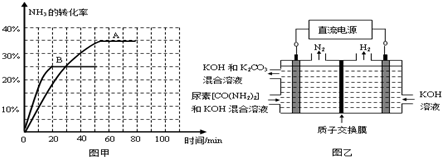
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��У�����ڶ����������ۻ�ѧ�Ծ��������棩 ���ͣ������
Ԫ�����ڱ��е�������Ԫ��������3d���ӵ�Ӱ�죬���ʵĵݱ�����������Ԫ�����в�ͬ��
��������Ԫ�صĵ�һ��������ԭ��������������������������ġ�
�أ�31Ga���Ļ�̬�����Ų�ʽ��_________________________________________��
31Ga�ĵ�һ������ȴ���Ե���30Zn��ԭ����______________________________________��
�������ڹ���Ԫ�ص������������γɶ��ֶ���������
��1��CO��NH3���Ժͺܶ���ɽ����γ�����CO��N2��Ϊ�ȵ����壬CO������Cԭ������һ�µ��Ӷԣ�C��Oԭ�Ӷ�����8�����ȶ��ṹ����CO�Ľṹʽ�ɱ�ʾΪ________________��NH3 ������Nԭ�ӵ��ӻ���ʽΪ_______�ӻ���NH3���ӵĿռ����幹����____________��
��2����ʢ������ͭˮ��Һ���Թ��мӰ�ˮ�������γ���ɫ�������������백ˮ�����ܽ⣬�õ�����ɫ����Һ�������Һ�м��Ҵ�����������ɫ���塣��ɫ�������ܽ⣬��������ԭ���ǣ�__________________________________________������ص����ӷ���ʽ�ͼ�����˵�����Խ��ͣ�
��3����ͼ����ʾΪ��άƽ�澧��ʾ��ͼ������ʾ�Ļ�ѧʽΪAX3����________��

��4��ͼ��Ϊһ������ͭ�ľ������˾���������ı߳�Ϊacm��Cu�����ԭ������Ϊ64������ͭ���ܶ�
Ϊ�� g/cm3�����ӵ������ɱ�ʾΪ________ mol-1(�ú�a���ѵĴ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com