��2010?ï����ģ����һ�������£��ϳ����е�������������ʼŨ�ȷֱ�Ϊa mol?L
-1��b mol?L
-1����ӦΪ��N
2+3H
2��2NH
3��������Ũ����ʱ��仯��ͼ1��ʾ��
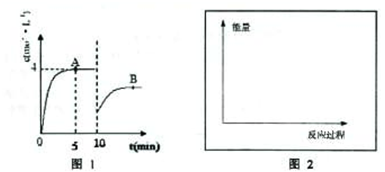
��1����Ӧ��5minʱ��������Ӧ����
1.2mol?L-1?min-1
1.2mol?L-1?min-1
��
��2����10minʱ��ȡ�Ĵ�ʩ��
��ȥ���ְ���
��ȥ���ְ���
��A��ƽ�ⳣ��Ϊ
����A��ƽ�ⳣ��K
=
=
�����������������=����B���ƽ�ⳣ����
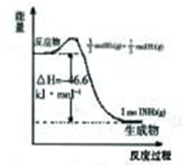
��3�����ϳ���������17g�����ų�45.5kJ��������ͼ2�����ϻ����úϳɰ���Ӧ������������ʱ��ı仯ʾ��ͼ��
��4��-50��Cʱ��Һ���������µ��룺2NH
3?NH
4++NH
-2��k=2��10
-2��Һ���ĵ���ﵽƽ��ʱ��������Ũ�ȴ�С��ϵΪ
c��NH3����c��NH+4��=c��NH-2��
c��NH3����c��NH+4��=c��NH-2��
������NH
4Cl���壬K
=
=
2��10
-12�����������������=����


 ��
�� ��
��

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�