
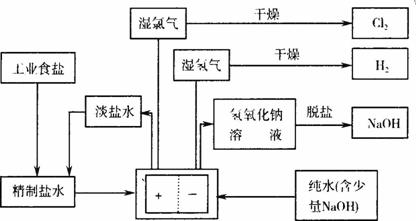
�ȼҵ�������ӽ���Ĥ������Ƽ����Ҫ��������ʾ��ͼ���£�
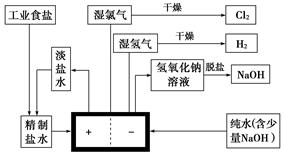
������ͼ���������գ�
(1)���Դ���������ĵ缫����������Ӧ�ĵ缫��ӦʽΪ____________________�����Դ���������ĵ缫��������Һ��pH________(����䡱�������ߡ����͡�)��
(2)��ҵʳ�κ�Ca2����Mg2����Fe3�������ʣ����ƹ����з�����Ӧ�����ӷ���ʽΪ_________________________________________________________________________��
(3)���������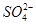 �����ϸߣ��������ӱ��Լ���ȥ
�����ϸߣ��������ӱ��Լ���ȥ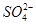 ���ñ��Լ�������________(����ĸ����)��
���ñ��Լ�������________(����ĸ����)��
a��Ba(OH)2���� b��Ba(NO3)2���� c��BaCl2
(4)Ϊ��Ч��ȥCa2����Mg2����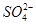 �������Լ��ĺ���˳��Ϊ________(����ĸ����)��
�������Լ��ĺ���˳��Ϊ________(����ĸ����)��
a���ȼ�NaOH�����Na2CO3���ټӱ��Լ�
b���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
c���ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��______����ȴ��______(��д��������)��ȥNaCl��
(6)��ͼʾ��֪�����ӽ���Ĥ������Ƽ����________��Ʒ��ѭ��ʹ�á�
(7)��֪NaCl��60 ����ܽ��Ϊ37.1 g���ֵ��60 �澫�Ʊ���ʳ��ˮ1371 g����������������Һ�ܶ�Ϊ1.37 g��cm��3�����к���20 g NaCl�������NaOH�����ʵ���Ũ��Ϊ________mol��L��1��
(1)2Cl����2e��===Cl2��������
(2)Ca2����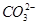 ===CaCO3����Mg2����2OH��===Mg(OH)2����Fe3����3OH��===Fe(OH)3��
===CaCO3����Mg2����2OH��===Mg(OH)2����Fe3����3OH��===Fe(OH)3��
(3)ac��(4)bc��(5)����������
(6)NaCl(����ˮ)��(7)7.14
��������
����������������ȼҵΪ����ͻ��������Ԫ�ؼ��仯������һ����֪ʶ��ͨ������Ľ����Ҫ��ȷ���¼��㣺(1)�ڵ�ⱥ��NaCl��Һʱ����������������Һ��pHΪʲô�����ߣ�������Ϊ�����ʱNa����H��������������H���ŵ�������Na��ǿ����H���������ŵ磺2H����2e��===H2������H�����������Ϸŵ������ˮ�ĵ���ƽ�⣬ʹ����������Һ��c(OH��)>c(H��)���ʵ��һ��ʱ�������������NaOH��ʹ��Һ��pH���ߡ�
(2)���ӽ���Ĥ������Ƽ�����ȼҵ��չ�ķ���ͬ��ǰ��Ĥ������Ƽ����ȱ�����Cl2������������NaOH��Һ�Ӵ��������·�Ӧ��Cl2��2NaOH===NaCl��NaClO��H2O��Ӱ���Ʒ��������
���㣺�����ȼҵ���жϡ����ʵij��ӡ�ʵ����������Լ���ѧ����
���������⿼��ѧ���ȼҵ���й�֪ʶ��Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�������Ŀ��ͨ����ij����Ҫ������Ʒ�Ĺ�ҵ��������ͼ�ķ����������ʵ��Ʊ������롢�ᴿΪ���������Ԫ�ػ�����֪ʶ��ʵ�������������ѧ��Ӧԭ������ѧ��ѧƽ��ԭ����ˮ��ԭ������ɫ��ѧ�۵㡢��ȫ������֪ʶΪ����Ŀ�꣬����Ϣ�Ļ�ȡ���ӹ������Ϻ����龳��ʵ������ķ������ۺϡ���������õ��ӽǷ���ʵ�������еĸ�������Ϊ��������Ŀ�ꡣ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͼ10-7
����ͼ10-7���������գ�
(1)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��________����ȴ��________(��д��������)��ȥNaCl��
(2)��ͼʾ��֪�����ӽ���Ĥ������Ƽ����________��Ʒ��ѭ��ʹ�á�
(3)��֪NaCl��
(��)�ڸ�Ĥ�����ʳ��ˮ(��ͼ10-8)�����У����Դ���������ĵ缫���������ķ�ӦΪ_________________________��
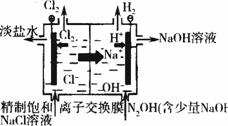
ͼ10-8 ���ӽ���Ĥ�����ԭ��ʾ��ͼ
���Դ���������ĵ缫������Һ��pH___________(����ߡ������½������䡱)����������Ĥ��������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����������NaClO��H2����Ӧ�Ļ�ѧ����ʽΪ______________________���ɸ�Ĥ�����ʳ��ˮ�����ӽ���Ĥ�Ĺ��ܣ����д����룬�ܷ��õ�ⱥ��Na2SO4��Һ�ķ�������NaOH��H2SO4?___________�����ܣ�����ͼ10-8�ڷ������Ի���װ��ʾ��ͼ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com