
��9�֣������������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
(1)��ҵұ�����Ļ�ѧ����ʽ��__________________________.
(2)��������������Һ��Ӧ�����ӷ���ʽ�� .
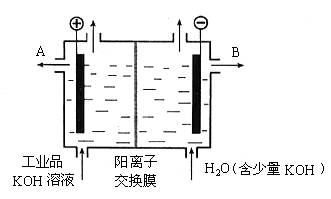
(3)��ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ.
�ٸõ��۵�������Ӧʽ��___________________.
��ͨ�翪ʼ������������ҺpH�����������ԭ��__________ .
�۳�ȥ���ʺ������������Һ�ӳ���______(��д��A����B��)������
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06�걱�����������������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
��1����ҵұ�����Ļ�ѧ����ʽ��__________________________��
��2����������������Һ��Ӧ�����ӷ���ʽ��__________________________��
��3����ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ��ֻ����������ͨ�������乤��ԭ����ͼ��ʾ��
�ٸõ��۵�������Ӧʽ��__________________________��
��ͨ�翪ʼ������������ҺpH�����������ԭ��____________________________
_______________________________________________________________________________________________________________________________________________��
�۳�ȥ���ʺ������������Һ��Һ������_____________����д��A����B����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��06�걱�����������������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
��1����ҵұ�����Ļ�ѧ����ʽ��__________________________��
��2����������������Һ��Ӧ�����ӷ���ʽ��__________________________��
��3����ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ��ֻ����������ͨ�������乤��ԭ����ͼ��ʾ��

�ٸõ��۵�������Ӧʽ��__________________________��
��ͨ�翪ʼ������������ҺpH�����������ԭ��____________________________
_______________________________________________________________________________________________________________________________________________��
�۳�ȥ���ʺ������������Һ��Һ������_____________����д��A����B����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)��ҵұ�����Ļ�ѧ����ʽ�� ��?
(2)��������������Һ��Ӧ�����ӷ���ʽ�� ��?
(3)��ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ����ͼ��ʾ��?
�ٸõ��۵�������Ӧʽ�� ��?
��ͨ�翪ʼ������������ҺpH�����������ԭ�� ��?
�� ��
�۳�ȥ���ʺ������������Һ��Һ����� (��д��A����B��)������?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.���ϡ������Һ��ʵ�����ǵ��ˮ������ҺpH����
B.���ϡ����������Һ��Ҫ����OH-������ҺpH��С
C.�����������Һ���������Ϻ�������������������ʵ���֮��Ϊ1��2
D.����Ȼ�ͭ��Һ���������Ϻ�������������������ʵ���֮��Ϊ1��1
(2)�����������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش�
�ٹ�ҵұ�����Ļ�ѧ����ʽ��______________________________________________��
����������������Һ��Ӧ�����ӷ���ʽ��___________________________________________��
�۹�ҵƷ�������ص���Һ�к���ijЩ����������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ(ֻ����������ͨ��)���乤��ԭ������ͼ��ʾ��

a.�õ��۵�������Ӧʽ��_________________________________________________��
b.ͨ�翪ʼ������������ҺpH�����������ԭ��________________________________��
c.��ȥ���ʺ������������Һ��Һ�����____________(��д��A����B��)������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com