
��12�֣����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ԭ�ӵĵ����Ų�ͼΪ |
| B | �����µ���Ϊ˫ԭ�ӷ��ӣ�ԭ�Ӽ��γ����Թ��õ��Ӷ� |
| C | ԭ�ӵ�s�������������p�����������Ԫ�ص������Ϊ-2�� |
| D | ������������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ |
| E | ԭ��������D������ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ���������еİ뵼����� |
| B | L��s��������p��������1 |
| C | ���ڵڶ����ڣ�ԭ������2��δ�ɶԵ��� |
| D | ǰ������ѽ����δ�ɶԵ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| A | ���������еİ뵼��Ŀ� |
| B | L��s��������p��������l |
| C | �ؿ��к�����ߵ�Ԫ�� |
| D | ������������Ԫ�������һ��������� |
| E | ����������δ�ɶԵ�������� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡƽ��ɽ�и߶���ѧ����ĩ���������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
[��ѧ����ѡ�����ʽṹ������]��30�֣�
��18�֣���ѡ�����и����з��������ѡ�
��1�� ���������У����ں��й��ۼ������Ӿ�����
A��CsCl B��KOH C��H2O D��H2
��2����֪CsCl������ܶ�Ϊ ��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ
��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ

A��  g/mol B��
g/mol B�� g/mol
g/mol
C��  g/mol D��
g/mol D�� g/mol
g/mol
��3����֪���������ͨʽXOm(OH)n����ʾ����X��S��m=2��n=2�������ʽ�Ӿͱ�ʾH2SO4��һ����ԣ���ʽ��m��ֵԽ�ú����������Խǿ�����и���������������ǿ����
A��HMnO4 B��H2SeO3 C��H3BO3 D��H3PO4
��12�֣����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
A |
ԭ�ӵĵ����Ų�ͼΪ |
|
B |
�����µ���Ϊ˫ԭ�ӷ��ӣ�ԭ�Ӽ��γ����Թ��õ��Ӷ� |
|
C |
ԭ�ӵ�s�������������p�����������Ԫ�ص������Ϊ-2�� |
|
D |
������������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ |
|
E |
ԭ��������D������ |
��������������ش��������⣺(����ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��
��3��E�����ڱ��е�λ���� ��ECl3����B��C���⻯���γ���λ��Ϊ6��������������������ʵ���֮��Ϊ2��1������������λ����磬ECl3�γɵ������Ļ�ѧʽΪ ��
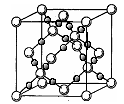
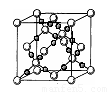
��4��AC2�ڸ��¸�ѹ�����γɵľ�������ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ������������壬�þ�����Aԭ�ӵ��ӻ���ʽΪ ��
��5��D �ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����11����ϰ��ѧ�Ծ� ���ͣ������
��12�֣����в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
A |
ԭ�ӵĵ����Ų�ͼΪ |
|
B |
�����µ���Ϊ˫ԭ�ӷ��ӣ�ԭ�Ӽ��γ����Թ��õ��Ӷ� |
|
C |
ԭ�ӵ�s�������������p�����������Ԫ�ص������Ϊ-2�� |
|
D |
������������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ |
|
E |
ԭ��������D������ |
��������������ش��������⣺(����ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
��1��A��B��C�ĵ縺����С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� �����⻯��ĵȵ������� ��
��3��E�ļ۵����Ų�ʽ�� ��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬ECl3�γɵ������Ļ�ѧʽΪ ��
��4��AC2�ڸ��¸�ѹ�����γɵľ�����ͼ��ʾ���þ������������ ��ѡ����ӡ�����ԭ�ӡ��������ӡ������������壬�þ�����Aԭ�ӵ��ӻ���ʽΪ ��

��5��D �ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
��13�֣�
���в���ǰ������Ԫ�ص����ʻ�ԭ�ӽṹ���±���
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
A |
���������еİ뵼��Ŀ� |
|
B |
L��s��������p��������l |
|
C |
�ؿ��к�����ߵ�Ԫ�� |
|
D |
������������Ԫ�������һ��������� |
|
E |
����������δ�ɶԵ�������� |
��1��д��Ԫ��E��̬ԭ�ӵĵ����Ų�ʽ�� ��
��2��C2���ʷ����У����� �� ����
��
����
�� ����Ԫ��B����̬�⻯�Ŀռ乹��Ϊ
��
����Ԫ��B����̬�⻯�Ŀռ乹��Ϊ
��
��3��A��B��C��һ�����ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ���� ��
��4��D���ʵ��۵� A���ʵ��۵㣨����ڡ����ڡ�������ԭ���ǣ�
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com