
��a����Ԫ�������������а����ŷdz���Ҫ�Ľ�ɫ������������Ҫ�Ľ������ϣ�
Ҳ������������Ԫ�ء�ÿ������ĸ�ʴ������ľ�����ʧ��ijУ�о�С����ʵ������ģ���������Ȼ�����µĸ�ʴ����������װ�ã������۲�����
��ش��������⣺
��1����Ƚ���������װ�����Ҳർ���к�īˮ�ĸ߶ȣ�____��____��____��
��д��Aװ���У�������ʴʱ������Ӧ�ĵ缫��Ӧʽ_____________________��
��2�������ķ������ش�����壬�����г���������Ʒ���ڸ��ﴦ����Ϳ�Ͽ��������Ա�
����������ӵ绯ѧ�Ƕ����һ�ַ���������ͼ�и������ⱻ��ʴ���뽫��ͼ��ɣ�
 |
��b��������һ�ֳ�������ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+ ��33%��36%����
����ˮ�������������е�θ�ᣬ��Vc��ά����C��ͬ�������ӱ�Ʒ���ա�ij��ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ��
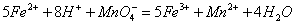
ȷ��������ҩƷ10.00g������ȫ�������Լ�1�У����Ƴ�1000mL��Һ��ȡ
��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��3����ʵ���е��Լ�1��______������ţ���
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
��4��������жϵζ��յ������___________________________________________��
��5����ͨ�����㣬˵����ҩƷ�����������Ƿ�ϸ�____________(��ϸ��ϸ�)
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A+��B����C2-��D��E��F��G��H�ֱ��ʾ����18�����ӵİ����������ӻ���ӣ��� ��ش�
��ش�
��1��AԪ����__________��BԪ����__________��CԪ����__________����Ԫ�ط��ű�ʾ����
��2��D��������Ԫ����ɵ�˫ԭ�ӷ��ӣ������ʽ��__________��
��3��E�����к�18�����ӵ���������������ǿ�ķ��ӣ������ʽ��__________��
��4��F��������Ԫ����ɵ���ԭ�ӷ��ӣ������ʽ��__________������ʽ��__________��
��5��G�����к���4��ԭ�ӣ������ʽ��__________��
��6��H�����к���8��ԭ�ӣ������ʽ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
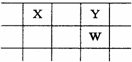
X��Y��Z��W��Q��ԭ������������������ֶ���������Ԫ�ء�����ֻ��Z�ǽ�����W�ĵ����ǻ�ɫ���壬X��Y��W�����ڱ��е����λ�ù�ϵ����ͼ������˵����ȷ����
A��Z��Q�γɵĻ�����ˮ��Һ��һ��������
B����̬�⻯���ȶ��ԣ�Y��W
C��Y�ĵ��ʱ�W�ĵ��ʷе��
D��ԭ�Ӱ뾶�Ӵ�С�����ǣ�Z��X��Y��W��Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з���ʽ��д��ȷ����
A��HCO3���ĵ��뷽��ʽ��HCO3��+ H2O  H2CO3 + OH��
H2CO3 + OH��
B��NH3��H2O�ĵ��뷽��ʽ��NH3��H2O NH4+ + OH��
NH4+ + OH��
C��CO32����ˮ�ⷽ��ʽ�� CO32�� + 2H2O H2CO3 + 2OH��
H2CO3 + 2OH��
D��NH4Cl��ˮ�ⷽ��ʽ��NH4+ + H2O===NH3��H2O + H+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A��CO(g)��ȼ���ȣ���H����283.0kJ/mol����2CO2(g) ��2CO(g)��O2(g)��Ӧ��
��H��+566.0kJ/mol
B����֪NaOH(aq)��HCl(aq)��NaCl(aq)��H2O(l) ��H����57.3 kJ��mol��1��
��40.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�57.3kJ������
C����֪2C(s)��2O2(g)��2CO2(g) ��H = a��2C(s)��O2(g)��2CO(g)����H = b����a��b
D����֪C (ʯī��s)��C (���ʯ��s) ��H��0����ʯī�Ƚ��ʯ�ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA���������ӵ�����������˵������ȷ����
A����״���£�11.2L�ȷ�(CHCl3)�к���C-Cl������ĿΪ1.5NA
B�����³�ѹ�£�15g��(-CH3)�����ĵ�����Ϊ9NA
C��ͬ��ͬѹ�£�1LNO��1LO2��ֻ�����С��1.5L
D��pH=l�Ĵ�����Һ100mL����������Ϊ0.01 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ӧ��
��Cl2��FeI2 FeCl2��I2
FeCl2��I2
��2Fe2����Br2 2Fe3����2Br��
2Fe3����2Br��
��Co2O3��6HCl 2CoCl2��Cl2����3H2O
2CoCl2��Cl2����3H2O
����˵����ȷ����
A����Ӧ�٢ڢ��е���������ֱ���I2��Fe3����CoCl2
B���������Ϸ���ʽ���Եõ������ԣ�Cl2��Fe3����Co2O3
C���ڷ�Ӧ���е�1 mol Co2O3�μӷ�Ӧʱ��2 mol HCl������
D�����������õ�Cl2��FeBr2 FeCl2��Br2
FeCl2��Br2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z���ֻ�������ɶ�����Ԫ����ɣ�����X��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ�������ֻ������������ת����ϵ(���ַ�Ӧ����P��Ӧ��������ȥ)��
��ش�
(1)X��Y����Һ�з�Ӧ�����ӷ���ʽΪ______________________________��
(2)������ͼװ��(�г̶ֹ�װ������ȥ)����ʵ�飬��װ�â��м���X��װ�â��м���һ�ֵ���ɫ�������ʽ��з�Ӧ��װ�â������ɰ�ɫ������װ�â��п��ռ���һ����ɫ���塣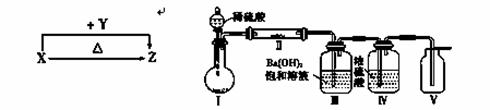
��װ�â��з�Ӧ�Ļ�ѧ����ʽΪ_____________________����������� ��
����װ�â������ʵķ�����____________________________________��
����X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ���������Ģ��е����壬��������װ����________(����ͼѡ���Ҫװ�ã���д���)��
��Ӧ�Ļ�ѧ����ʽΪ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У�����ȡ����Ӧ����( )
��CH3CH=CH2+Br2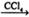 CH3CHBrCH2Br ��CH3CH2OH
CH3CHBrCH2Br ��CH3CH2OH 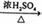 CH2=CH2+H2O
CH2=CH2+H2O
��CH3COOH+CH3CH2OH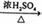 CH3COOCH2CH3+H2O ��2CH3CH2OH
CH3COOCH2CH3+H2O ��2CH3CH2OH 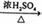 CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
A. �٢� B.�ۢ� C.�٢� D.�ڢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com