
��12�֣�ij��ѧ��ȤС����̼��Ũ����Ϊ��ʼԭ�ϣ�������һ�Ȼ�����������Ʒ�Ӧ�Ʊ��������ơ����װ�����£�������װ���п�����Ӱ�죩
��ش��������⣺
��1��װ��A���Թ��з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2���²�B�п��Թ۲����Ҫ������
��Cƿ������������ ��
��3����֪���������´��ڷ�Ӧ3HNO2=HNO3+2NO��+H2������������Һ�У�NO![]() �ɽ�MnO
�ɽ�MnO![]() ��ԭΪMn2+�����������ɡ�д������D�в������������Ƶķ�����
��ԭΪMn2+�����������ɡ�д������D�в������������Ƶķ�����
��
��4��Eװ�õ������� ��
��5��ͬѧ�Ǿ���������Ϊ����װ������ȱ�ݣ�Ϊ����D��������NaOH������ΪӦ�ý��еĸĽ��� ��
��1��C+4HNO3��Ũ��CO2��+4NO2��+2H2O
��2����Һ������NO����һ����������
��3������I���������������Թ��У�����ϡ���ᣬ��������ɫ���岢��Һ���Ϸ���Ϊ����ɫ����D�в������������ơ���Ӧ�����ӷ���ʽ��3NO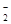 +2H+=NO
+2H+=NO![]() +2NO��+H2O
+2NO��+H2O
����II���������������Թ��У���������KMnO4��Һ������Һ��ɫ��ȥ����D�в������������ƣ���Ӧ���뷽��ʽ��5 NO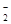 +2MnO
+2MnO![]() +6H+=5 NO
+6H+=5 NO![]() +2Mn2++3H2o�����������𰸾��÷֣�
+2Mn2++3H2o�����������𰸾��÷֣�
��4������D��δ��Ӧ���NO��������Ⱦ���������������𰸾��÷֣�
��5����C��D֮������һ��ʢŨ�����ϴ��ƿ����װ��ˮCaCl2�ĸ���ܣ������������𰸾��÷֣�
����:



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ�����и�����ѧ����ĩ��ѧ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
�������ƹ㷺����ӡȾ��Ư����ҵ���ڽ�����ҵ��������������ʳƷ��ҵ������������ɫ��������һ��DZ���°����ʣ���������ʳ�ö��˲���Σ��������������������ζ�������NaCl���ƣ�����η�������ʳ����ʳ���¼���ij��ѧ��ȤС����̼��Ũ����Ϊ��ʼԭ�ϣ��������װ�ã�����һ��������������Ʒ�Ӧ�Ʊ��������ơ����г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩��

�������ϣ���HNO2Ϊ���ᣬ�����´��ڷ�Ӧ3HNO2��HNO3��2NO����H2O��
��NO2���ܱ��ܶೣ����ǿ����������������������Һ�пɽ�MnO4����ԭΪMn2�������������ɡ�
��NO����Ӧ���ɱ�����KMnO4��Һ����Ϊ����
��HNO2��������ҺҲ��һ�������������ܰ�I��������I2��
��AgNO2��һ��������ˮ��������İ�ɫ�����
��1��д��C���Ʊ��������Ʒ�Ӧ�Ļ�ѧ����ʽ(��Ӧ������ʵ���֮��Ϊ2��1)??????? ��
��2��Dװ�õ�������??????? ��
��3��������C�������������ƺ������١���ͬѧ��ΪC�в��ﲻ�����������ƣ�����̼���ƺ��������ơ�Ϊ�ų����ż�ͬѧ��B��Cװ�ü�����װ��E��E��ʢ�ŵ��Լ�Ӧ��________��д���ƣ���
��4������������Ѫ�쵰���к�Fe2+������ʳ�������Σ���NaNO2��������Ѫ�쵰����Fe2+ת��ΪFe3+���ж�,����ά����C�ɽⶾ��������������ȷ����?????? ��
A�����������ǻ�ԭ��????????????? ????????????? ????????????? ? B��ά����C�ǻ�ԭ��
C��ά����C��Fe3+��ԭΪFe2+????????????? ????????????? ????????????? ? D���������α���ԭ
��5������������NaNO2��NaCl�ķ�����?????????
A ������ҺpH��
B�����ᷨ
C��AgNO3��HNO3�����Լ���??
D����KI���ۣ����ԣ���
E���������ַ�������
��6��ijͬѧ����ˮ�ӵ�NaNO2��Һ�۲쵽��ˮ��ɫ����д���˷�Ӧ�����ӷ���ʽ??????????????????????????????????????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʯ��ɽ��2010�����һģ�������ۺϣ���ѧ���� ���ͣ������
��12�֣�ij��ѧ��ȤС����̼��Ũ����Ϊ��ʼԭ�ϣ�������һ�Ȼ�����������Ʒ�Ӧ�Ʊ��������ơ����װ�����£�������װ���п�����Ӱ�죩

��ش��������⣺
��1��װ��A���Թ��з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2���²�B�п��Թ۲����Ҫ������
��Cƿ������������ ��
��3����֪���������´��ڷ�Ӧ3HNO2=HNO3+2NO��+H2������������Һ�У�NO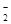 �ɽ�MnO
�ɽ�MnO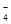 ��ԭΪMn2+�����������ɡ�д������D�в������������Ƶķ�����
��ԭΪMn2+�����������ɡ�д������D�в������������Ƶķ�����
��
��4��Eװ�õ������� ��
��5��ͬѧ�Ǿ���������Ϊ����װ������ȱ�ݣ�Ϊ����D��������NaOH������ΪӦ�ý��еĸĽ��� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com