
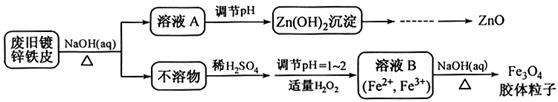
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
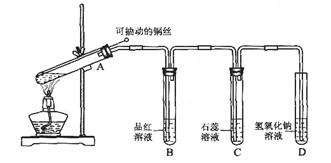
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢܢ� | B���ڢۢ� | C���٢ڢ� | D���ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
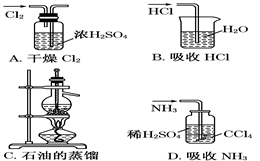
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ� |
| B�����з�Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| C�����������ᾧ����ʱ��Ӧ����Һ���� |
| D�����г�������ʱ��Ӧ�����������������ƽ�����̣��������������ƽ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ��ʱ����ˮ����Ũ��������ϡ���� |
| B����ɫ��Ӧʵ��ǰ����˿Ӧ��������ϴ�����ھƾ�������������û����ɫ |
| C��ʵ���У�ȡ���ƺ��ʣ�ಿ�ֿ��ԷŻ�ԭ�Լ�ƿ�� |
| D����Һ����ʱ���Ƚ���Һ©�����²�Һ����¿ڷų����ٽ��ϲ�Һ����Ͽڵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������20 mL | B����20 mL | C������20 mL | D������5mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
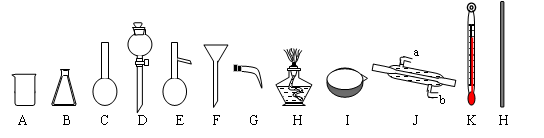
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com