
̼����ĺ���Ӱ��������ܡ�̼��������һ�ֲⶨ�����ǽ������е�̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
(1)����װ��A���ڸ����½�x g�����е�̼����ת��ΪCO2��SO2��

������a�ijɷ���______��
��������������FeS��ʽ���ڣ�A�з�Ӧ��
3FeS��5O2 1________��3________��
1________��3________��
(2)������aͨ�����װ����(��ͼ)�����õζ����ⶨ��ĺ�����

��H2O2����SO2�Ļ�ѧ����ʽ��__________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mL NaOH��Һ��������1 mL NaOH��Һ�൱���������Ϊy g����ø������������������________��
(3)������aͨ���̼װ����(��ͼ)�������������ⶨ̼�ĺ�����

������aͨ��B��C��Ŀ����________________________________________��
�ڼ��������̼������������Ӧ������������_______________________________________________________��
(1)��O2��SO2��CO2����Fe3O4��SO2��
(2)��H2O2��SO2===H2SO4����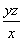 ��
��
(3)���ų�SO2��CO2�ⶨ�ĸ��š�������CO2ǰ��������ƿ������
[����] (1)�ٸ����������գ�̼����ת��Ϊ������̼�Ͷ���������������ɷ�ΪCO2��SO2��O2��������Ԫ�صĴ�����ʽΪFeS�����ݸ����Ļ�ѧ��������3���������ΪSO2������������غ�ȷ��1���������ΪFe3O4����ѧ����ʽΪ3FeS��5O2 Fe3O4��3SO2��
Fe3O4��3SO2��
(2)��H2O2��SO2��Ӧ�Ļ�ѧ����ʽΪH2O2�� SO2=== H2SO4���ڸ�������1 mL������������Һ�൱������y g��������z mL������������Һ�൱�ں�����Ϊzy g�������������Ϊ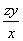 ��
��
(3)�����мȺ��ж��������ֺ��ж�����̼���ⶨ������̼ǰ�����ȥ������������ţ�����B��Cװ�����ڳ�ȥ������̼�еĶ������ⶨ̼�ĺ������ⶨ������̼�����������Ҫ�ⶨ�����������ն�����̼װ��(������̼����ƿ)ǰ�������(������ֵΪ������̼������)��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�������ʺ�ij��Һ��һ�������·�Ӧ�������������Է�������(��ƽ����Է�������)Ϊ45��������Ӧ������һ����������
A��Zn��ŨH2SO4 B Na2O2��NH4ClŨ��ҺC��Cu��ŨHNO3 D��C��ŨHNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������N2H4��������������һ����Ҫ�Ļ��ȼ�ϡ�N2H4��N2O4��Ӧ�ܷų��������ȡ�
��1����֪��2NO2(g)  N2O4(g) ��H=-57.20 kJ��mol-1��һ�������£������һ�����ܱ������з�Ӧ2NO2(g)
N2O4(g) ��H=-57.20 kJ��mol-1��һ�������£������һ�����ܱ������з�Ӧ2NO2(g) N2O4(g)�ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ��� (����ĸ����
N2O4(g)�ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ��� (����ĸ����
A����СNO2��Ũ�� B�������¶� C������NO2��Ũ�� D�������¶�
��2��25��ʱ��1.00 g N2H4(l)������N2O4(l)��ȫ��Ӧ����N2(g)��H2O(l)���ų�19.14 kJ����������÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3��17�桢1.01��105 Pa���ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ��c(NO2)=0.0300 mol��L-1��c(N2O4)=0.0120 mol��L-1����Ӧ2NO2(g) N2O4(g)��ƽ�ⳣ��K=_________
N2O4(g)��ƽ�ⳣ��K=_________
��4������һ������Cu��������ŨHNO3��Ӧ���Ƶ�1.00 L�Ѵﵽƽ���N2O4��NO2�Ļ�����壨17�桢1.01��105 Pa��������������������Cu________ g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
(1)A�����ڱ��е�λ����________��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ��__________________________��
(2)B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ__________����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________________���ڲ����м�������KI����Ӧ�����CCl4�����л�����______ɫ��
(3)����ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ������A�Ķ�Ԫ���ӻ����� |
| b | �����зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| c | ����ѧ���ΪBDF2 |
| d | ��ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
a�Ļ�ѧʽΪ________��b�Ļ�ѧʽΪ______________��c�ĵ���ʽΪ________��d�ľ���������________��
(4)��A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ��________�����ɾ��п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������(Na2S2O3)�����������Լ������ﻹԭ���������ȡ������ֽ⡣��ҵ�Ͽ��÷�Ӧ��2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2�Ƶá�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��

�ش��������⣺
(1)b�з�Ӧ�����ӷ���ʽΪ________________��c���Լ�Ϊ________��
(2)��Ӧ��ʼ��c�����л��Dz��������ֱ���塣�˻�������________��
(3)d�е��Լ�Ϊ________��
(4)ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ��______________________________________(д������)��
(5)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2���ܹ�����ԭ����______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���еݱ���ɲ���ȷ����( )
A��Na��Mg��Al��ԭ�����μ��� B�� C��N��Oԭ�Ӱ뾶��������
C��I2��Br2��Cl2������������ǿ D��P��S��Cl���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��Ϊ��������X��ϡ����ķ�Ӧ�У���������Z����������Һʱ����ʹ��Ӧ�ӿ죬X��Y���ԭ���ʱ��Y�缫�������٣�X��Y��Z���ֽ����Ļ˳��Ϊ�� ��
A X>Y>Z B X>Z>Y C Y>X>Z D Y>Z>X
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Al2O3Ϊԭ����ȡ������������õķ�����(����)
A����Al2O3����ˮ
B����Al2O3�����������У�֮��μ�����������Һ
C����Al2O3�����������У�֮��μӰ�ˮ
D����Al2O3������NaOH��Һ�У�֮��μ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʱ��������ʳ���Ͼƿ���ʹ���Ƶ�������������ζ��������ζ������
A��ʳ�� B��ʳ���е�����
C�Ͼ��е��Ҵ� D���Ͼ��е��Ҵ���ʳ���е����ᷴӦ���ɵ���������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com