
A. ����ˮ�棬�۳�������С���ı��ζ�������С����Һ���ɫ
B. �������������壬�����ȼ����Һ��dz��ɫ
C. ��Ӧ���־��ң������������ȼ
D. ���ҷ�Ӧ�������������ȼ
E. ���ɰ�ɫ��״�������̶�������ʧ
F. ���ɵ���ɫ����
��3��ʵ�����ݣ�
ʵ�鷽���������������������������������� ʵ�������������������������������������� �йػ�ѧ����
�������������������������������������������� ��������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��4��ʵ����ۣ�________��
��5���������ۣ�����ӽṹ�����ϼ�˵���������۵�ԭ��
�����㲹��һ��ʵ�鷽���������У���֤��������������������Ԫ�ص����ʵݱ���ɡ�
�������ж�����Ԫ�����ʵ����ݣ�
Ԫ�ر��
Ԫ�������������� ���������� ���������� ���������� ���������� ���������� ���������� ���������� ��
ԭ�Ӱ뾶
��10-10m������ 0.74�������� 1.60�������� 1.52�������� 1.10�������� 0.99�������� 1.86�������� 0.75�������� 0.82
��ߺ���
�ͻ��ϼ������������������������� +2�������� +1�������� +5�������� +7�������� +1�������� +5�������� +3
�������������������� -2���������������������������������������� -3�������� -1������������������������ -3��������
�Իش��������⣺
��1������Ԫ���д���ͬһ�������________��Ԫ�آ������ڱ��е�λ��Ϊ________��
��2���ϱ���ij����Ԫ�أ��γɵķ����У�ÿ��ԭ�Ӷ����������Ϊ8���ӵ��ȶ��ṹ��д�������ʽ________��
��3��Ԫ�آ٢����γ����ֻ����д�����н��ȶ��Ļ���������ˮ��Ӧ�ģ����ӷ���ʽ��________��
| ��1����֤��������Ԫ�ش����ҽ����Ժͷǽ����Եĵݱ���ɡ�
��2���������Թܡ��ձ����ƾ��ơ���ֽ��ɰֽ�����ӣ�С������ͷ�ιܡ���� �Լ����ơ�þ������Ƭ��������ˮ�����Ʊ���H2S��Һ2mol��L��1���ᡢNaOH��Һ������ˮ����̪��Һ��AlCl3��Һ�� ��3��1 B Mg��2H2O=Mg��OH��2��H2�� 2 F H2S��Cl2=2HCl��S�� 3 A 2Na��2H2O=2NaOH��H2�� 4 D Mg��2HCl=MgCl2��H2�� 5 C 2Al��6HCl=2AlCl3��3H2�� 6 E AlCl3��3NaOH=Al��OH��3����3NaCl Al��OH��3��NaOH=NaAlO2��2H2O ��4��Na��Mg��Al��S��ClԪ�أ�����ԭ�������ĵ�����Ԫ�صĽ������������ǽ���������ǿ�� ��5����ͬһ����Ԫ�ش���������ԭ�������ĵ�����ԭ�Ӱ뾶��С���˶������ӵ�����������ǿ�����ʧ���ӵ������������õ��ӵ���������ǿ��Ԫ�صĽ������������ǽ���������ǿ�� ��������δ��֤��Ԫ��ΪSi��P�������������ʵ��ȷ����ǽ�����ǿ������Na2SiO3��Һ�еμ�H3PO4��Һ����������ɫ��״��������˵��H3PO4��H2SiO3����ǿ���Ӷ�֤����Ԫ�صķǽ����Աȹ�ǿ����������������Ҳ�ɣ�
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ����� | ʵ������ |
| 1����ɰֽ�����þ�����ˮ��Ӧ������Ӧ����Һ�еμӷ�̪ | ��A������ˮ�棬�۳�һ��С����ˮ���������� ������֮��ʧ����Һ���ɫ ��B���������壬���ڿ�����ȼ�գ���Һ���dz��ɫ ��C����Ӧ��ʮ��ǿ�ң���������������ڿ�����ȼ �� ��D�����ҷ�Ӧ��������ȼ������ ��E����Һ�ļ������� ��F�����ɵ���ɫ���� |
| 2�������Ƶ�H2S������Һ�еμ����Ƶ���ˮ | |
| 3��������з�̪��Һ����ˮ��Ӧ | |
| 4��þ����2mol��L-1�����ᷴӦ | |
| 5��������2mol��L-1�����ᷴӦ | |
| 6���ⶨ 0.1mol/L��Na2SiO3��Na3PO4��Na2SO4��Һ��pH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鷽�� | ʵ������ |
| 1����ɰֽ�����þ�����ˮ��Ӧ������ӦҺ�еμӷ�̪ 2�������Ƶ�H2S������Һ�еμ����Ƶ���ˮ 3��������з�̪��Һ����ˮ��Ӧ 4��þ����2mol/L�����ᷴӦ 5��������2mol/L�����ᷴӦ 6����AlCl3��Һ�еμ�NaOH��Һ������ |
A������ˮ�棬�۳�С���Ĵ��ζ�������С����Һ���ɫ B�������������壬�����ȼ����Һ��dz��ɫ C����Ӧ��ʮ��ǿ�ң������������ȼ D�����ҷ�Ӧ�������������ȼ E�����ɰ�ɫ��״�������̶�������ʧ F�����ɵ���ɫ���� |
| ʵ�鷽�� | ʵ������ | �й����ӷ���ʽ |
| A | ||
| B | �� �� | |
| �� �� | ||
| �� �� | ||
| E | ||
| F |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
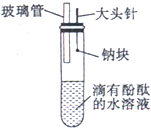 ijͬѧ��ͬ����Ԫ�����ʵݱ����ʵ��ʱ���Լ������һ��ʵ�鷽��������¼���й�ʵ���������±���
ijͬѧ��ͬ����Ԫ�����ʵݱ����ʵ��ʱ���Լ������һ��ʵ�鷽��������¼���й�ʵ���������±���| ʵ�鷽�� | ʵ������ |
| 1��ɰֽ�����þ�����ˮ��Ӧ������Ӧ����Һ�еμӷ�̪ | A����ˮ�棬�۳�һ��С����ˮ���������ƶ�����֮��ʧ����Һ���ɫ |
| 2�����Ƶ�H2S������Һ�еμ����Ƶ���ˮ | B�������壬���ڿ�����ȼ�գ���Һ���dz��ɫ |
| 3������з�̪��Һ����ˮ��Ӧ | C��Ӧ��ʮ��ǿ�ң���������������ڿ�����ȼ�� |
| 4þ����2mol?L-1�����ᷴӦ | D���ҷ�Ӧ��������ȼ������ |
| 5������2mol?L-1�����ᷴӦ | E���ɰ�ɫ��״�������ȶ�������ʧ |
| 6��AlCl3��Һ�μ�NaOH��Һ������ | F���ɵ���ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
��ɰֽ�����þ��ؤ��ˮ��Ӧ������Ӧ����Һ�еμӷ�̪��Һ�����Ƶ�H2S������Һ�еμ����Ƶ���ˮ��������з�̪����ˮ��Ӧþ����2mol��L-1���ᷴӦ������2mol��L-1���ᷴӦ��ALCI3��Һ�еμ�NaOH��Һ������
A. ����ˮ�棬�۳�������С���ı��ζ�������С����Һ���ɫ
B. �������������壬�����ȼ����Һ��dz��ɫ
C. ��Ӧ���־��ң������������ȼ
D. ���ҷ�Ӧ�������������ȼ
E. ���ɰ�ɫ��״�������̶�������ʧ
F. ���ɵ���ɫ����
��3��ʵ�����ݣ�
ʵ�鷽���������������������������������� ʵ�������������������������������������� �йػ�ѧ����
�������������������������������������������� ��������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��4��ʵ����ۣ�________��
��5���������ۣ�����ӽṹ�����ϼ�˵���������۵�ԭ��
�����㲹��һ��ʵ�鷽���������У���֤��������������������Ԫ�ص����ʵݱ���ɡ�
�������ж�����Ԫ�����ʵ����ݣ�
Ԫ�ر��
Ԫ�������������� ���������� ���������� ���������� ���������� ���������� ���������� ���������� ��
ԭ�Ӱ뾶
��10-10m������ 0.74�������� 1.60�������� 1.52�������� 1.10�������� 0.99�������� 1.86�������� 0.75�������� 0.82
��ߺ���
�ͻ��ϼ������������������������� +2�������� +1�������� +5�������� +7�������� +1�������� +5�������� +3
�������������������� -2���������������������������������������� -3�������� -1������������������������ -3��������
�Իش��������⣺
��1������Ԫ���д���ͬһ�������________��Ԫ�آ������ڱ��е�λ��Ϊ________��
��2���ϱ���ij����Ԫ�أ��γɵķ����У�ÿ��ԭ�Ӷ����������Ϊ8���ӵ��ȶ��ṹ��д�������ʽ________��
��3��Ԫ�آ٢����γ����ֻ����д�����н��ȶ��Ļ���������ˮ��Ӧ�ģ����ӷ���ʽ��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com