
����Ŀ����CO2��H2Ϊԭ����ȡ�Ҵ��ķ�ӦΪ 2CO2(g)+6H2(g)CH3CH2OH(g)+3H2O(g)����H<0��ijѹǿ�� ���ܱ�������,��CO2��H2�����ʵ�����Ϊ 1��3 Ͷ��,��ͬ�¶���,�ﵽƽ���ƽ����ϵ�и����ʵ����ʵ�������(y%)���¶ȱ仯��ͼ��ʾ������˵����ȷ����
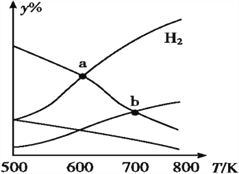
A. a���ƽ�ⳣ��С��b�� B. b��:v��(CO2)=v��(H2O)
C. a��:n(H2)=n(H2O) D. �¶Ȳ���,�������H2,v(CO2)ʼ�ղ���
���𰸡�C
�����������������A����ͼ���֪���¶�Խ�������ĺ���Խ��˵����Ӧ���淴Ӧ�����ƶ���������Ӧ�����Ƿ��ȣ��¶����ߣ�ƽ��Ũ�ȼ�С����ƽ�ⳣ��a>b��A����B��b��ֻ��˵�Ǹ��¶��£�CO2��H2O��Ũ����ͬ��b��ʱ��Ӧ����Ȼ��С����������Ȼ����˵��ƽ��������Ӧ������У�����v����CO2����v�棨H2O����B����C������ͼ�������a��ΪH2��H2O���ʵ����Ľ��㣬������ȣ�C��ȷ��D�����������㶨���������H2������Ӧ���Ũ��������Ӧ������������v��CO2��Ҳ����D����ѡC��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����MgSO4��Al2(SO4)3�Ļ����Һ�У���μ���NaOH��Һ������ͼ���У�����ȷ��ʾ������Ӧ����(�������ʾ����NaOH��Һ��������������ʾ��Ӧ���ɳ���������) (����)

A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ҫ�ɷ�ΪAl2O3�������������ʣ�����ȡ���Ĺ������̼��������£�

�Իش��������⣺
��1���Լ�XΪ__________��
��2������������������Ϊ_____________����������ƣ�����ʵ���ҽ��иò���ʱ��Ҫ�IJ���������__________________________________��
��3��������������������ڸ����£��ᷢ�����ҵķ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________�����һ���÷�Ӧ����;________________________��
��4����Ӧ�������ӷ���ʽΪ____________________________________________________��
��5�����������������ȡ������������0.6 mol���ӷ���ת�ƣ��������ܵõ���������������________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ܱ������У���ӦaA(��)![]() bB(��)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ��ƽ��ʱ��B��Ũ����ԭ����60%����
bB(��)�ﵽƽ������¶Ȳ��䣬�������������һ�������ﵽ��ƽ��ʱ��B��Ũ����ԭ����60%����
A. ƽ�����淴Ӧ�����ƶ��� B. ����A��ת���ʼ�С��
C. ����B���������������� D. a>b
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe��CO��Ni��Ϊ�ڢ���Ԫ�أ����ǵĻ��������������������Ź㷺��Ӧ�á�
��1����̬COԭ�ӵļ۵����Ų�ʽΪ____________��Co3+����3d�ܼ�����_________�ԳɶԵ��ӡ�
��2��Co3+��һ��������[Co(N3)(NH3)5]2+�У�Co+ ����λ����______________��1mol �������������Ҽ�����ĿΪ_____________����λ��N3-����ԭ���ӻ�����Ϊ____________________��
��3��Co2+��ˮ��Һ����[Co(H2O)6]2+���ڡ���Co2+����Һ�м��������ˮ�����ɸ��ȶ���[Co(NH3)6]2+ ����ԭ����___________________��
��4��ij��ɫ�����У�Fe2+��Fe3+�ֱ�ռ�������廥�����ڵĶ��㣬���������ÿ�����Ͼ���һ��CN-��K+λ���������ijǡ��λ���ϡ��ݴ˿�֪�þ���Ļ�ѧʽΪ____________����������Fe2+�����������γɵĿռ乹����_____________________��
��5��NiO�ľ���ṹ��ͼ����ʾ�����������������A Ϊ(0,0,0)��BΪ(1,1,0)����C�����������Ϊ_______________��

��6��һ���¶��£�NiO��������Է��ط�ɢ���γɡ������Ӳ㡱��������ΪO2-�����õ������У�Ni2+������У���ͼ�ң�����֪O2-�İ뾶Ϊa pm��ÿƽ��������Ϸ�ɢ�ĸþ��������Ϊ__________g���ú�a��NA�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ���䷴Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2==3Na2S2O3+CO2���÷�Ӧ��H>0����ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O���������¡�

��1������װ����ͼ��ʾ��

��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ��� ____________ ������SO2����Ч�ʵ͵�ʵ��������B����Һ _________________��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ�� __________________ ������һ����
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH��10.2����
��� | ʵ����� | Ԥ������ | ���� |
�� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬ _________�� | �а�ɫ�������� | ��Ʒ��NaCl |
�� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬________�� | �а�ɫ�������ɣ��ϲ���ҺpH>10.2 | ��Ʒ��NaOH |
��3��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�
��һ����ȷ��ȡa g KIO3����Է�������Ϊ214�����������Һ��
�ڶ������������KI�����H2SO4��Һ���μ�ָʾ����
�������� ��Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ�����ΪV mL��
��c(Na2S2O3)��_________mol��L��1��
��֪��IO3-+5I-+6H+= 3I2��3H2O ��2S2O32����I2=S4O62����2I��
��4��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�Na2S2O3Ũ�ȿ���__________����������Ӱ��������ƫ��������ƫ��������ԭ����_________________________________���������ӷ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڻ�ҩ���ҹ����Ĵ���֮һ���������˳��ޣ��䷴Ӧԭ��Ϊ��
S+3C+2KNO3![]() N2��+3CO2��+K2S
N2��+3CO2��+K2S
��ش�����������⣺
��1��������0.1molN2ʱ����Ӧ�й�ת�Ƶ�����Ϊ_____����ԭ�������ʵ���Ϊ_____mol��
��2�������ɱ�״����33.6LCO2����S������C�����ʵ�����_____mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(CH3COOOH)����ɫ��Һ�壬������ˮ���ӷ��������ֽ⣬����ǿ�����Եĸ�Ч��������ʹ�ù�������������ʱͨ������ˮϡ��ҵƷ�������ᣬȻ�����������Կ�����������������
��1��ijѧ����ʵ�������ܶ�Ϊ1.15g/mL����������Ϊ15%�Ĺ�ҵƷ������������0.1mol/L����������Һ250mL����������Ͳ��ȡ��ҵƷ�����������___________________ mL������Ͳ�����������ձ����Ҫ������������__________________________________________________________________________��
��2����ѧ������ǰ��������в�����������IJ���˳����___________________________________________����ĸ��ʾ��ÿ����ĸֻ����һ�Σ���
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ��������Ĺ�ҵƷ�������ᣬ�ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�Ĺ�ҵƷ���������ز�����ע������ƿ��
D��������ƿ�ǽ������ò���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죨ѡ����ƫ���� ���� ƫ����������Ӱ������?
a. ����ƿ������ˮϴ�Ӻ�δ���������������ˮ_____________________��
b. ת����Һʱ��������������Һ��������ƿ��___________________________��
c����ȡ15%�Ĺ�ҵƷ��������ʱ��������Ͳ___________________________________��
d�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ����伸��ˮ���̶���____________��
��4������������ȷ����__________________(����ĸ)��
A.���ù��˵ķ���������������Һ�л��е�NaCl����
B.���������װ��Ӧ����Σ�վ����ǩӦ����ͼ��ʾ
C.��������Ӧע���ܱա����±�������ɫϸ��ƿ��
D.����������һ���л��������������ȡ��ˮ�еĵⵥ��
��5�����������Сʱ�ڻ���ȫ�ֽ������(CH3COOH)��һ�ֳ��������嵥�ʣ���������嵥�ʵ�ʵ�鷽����_________________________________________________________________________________________��
��6������������������ԭ��CH3COONa�л�����SO42��,Ҫ�����SO42����ѡ�������Լ����ռ����Ⱥ�˳�����_________________________���Լ�����ѡ�꣬����ţ���
������ڴ��ᱵ��Һ���Ȼ�����Һ�ܴ����̼������Һ��̼��������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£����и���������һ��ʵ��ͼ����ʾת����ϵ����

ѡ�� | X | Y | Z | W |
A | Al | Al2O3 | NaAlO2 | Al(OH)3 |
B | Fe3O4 | Fe | FeCl2 | FeCl3 |
C | H2SO4 | SO2 | S | SO3 |
D | CH3CH2Br | CH2=CH2 | C2H5OH | CH2BrCH2Br |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com