
ФГЕиУКэЗЪЏОдЄДІРэКѓКЌSiO2ЃЈ63%ЃЉЁЂAl2O3ЃЈ25%ЃЉЁЂFe2O3ЃЈ5%ЃЉМАЩйСПИЦУОЕФЛЏКЯЮяЕШЃЌвЛжжзлКЯРћгУЙЄвеЩшМЦШчЯТЃК
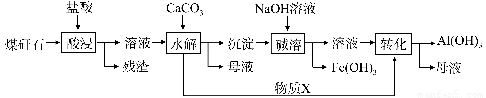
ЃЈ1ЃЉЁАЫсНўЁБЙ§ГЬжажївЊЗДгІЕФРызгЗНГЬЪНЮЊ_____________ЁЂ_________________ЁЃ
ЃЈ2ЃЉЁАЫсНўЁБЪБТСНўГіТЪЕФгАЯьвђЫиПЩФмга_____________ЁЂ___________ЁЃЃЈаДГіСНИіЃЉ
ЃЈ3ЃЉЮяжЪXЕФЛЏбЇЪНЮЊ___________ЁЃЁАМюШмЁБЪБЗДгІЕФРызгЗНГЬЪНЮЊ____________ЁЃ
ЃЈ4ЃЉвбжЊFe3+ПЊЪМГСЕэКЭГСЕэЭъШЋЕФpHЗжБ№ЮЊ2.1КЭ3.2ЃЌAl3+ПЊЪМГСЕэКЭГСЕэЭъШЋЕФpHЗжБ№ЮЊ4.1КЭ5.4ЁЃЮЊСЫЛёЕУВњЦЗAlЃЈOHЃЉ 3ЃЌДгУКэЗЪЏЕФбЮЫсНўШЁвКПЊЪМЃЌШєжЛгУCaCO3вЛжжЪдМСЃЌКѓајВйзїЙ§ГЬЪЧ____________________ЁЃ
ЃЈ5ЃЉвдУКэЗЪЏЮЊдСЯЛЙПЩвдПЊЗЂЦфЫћВњЦЗЃЌР§ШчдкУКэЗЪЏЕФбЮЫсНўШЁвКГ§ЬњКѓЃЌГЃЮТЯТЯђAlCl3ШмвКжаВЛЖЯЭЈШыHClЦјЬхЃЌПЩЮіГіДѓСПAlCl3ЁЄ6H2OОЇЬхЃЌНсКЯЛЏбЇЦНКтвЦЖЏдРэНтЪЭЮіГіОЇЬхЕФдвђЃК_______________________ЁЃ
ЃЈ1ЃЉAl2O3+6H+==2Al3++3H2O? ЃЛ? Fe2O3+6H+==2Fe3++3H2O??? ЃЈ4ЗжЃЌУПИі2ЗжЃЉ
ЃЈ2ЃЉбЮЫсЕФХЈЖШЁЂЗДгІЮТЖШЁЂУКэЗЪЏПХСЃДѓаЁЁЂЪЧЗёГфЗжНСАшЁЂЗДгІЪБМфЃЈШЮаДСНИіЃЉЃЈ4ЗжЃЌУПИі2ЗжЃЉ
ЃЈ3ЃЉCO2 ЃЛ AlЃЈOHЃЉ3 +OHЁЊ=AlO2ЁЊ+2H2O???? ЃЈ4ЗжЃЌУПИі2ЗжЃЉ
ЃЈ4ЃЉМгШыCaCO3ЕїНкpHЕН3.2ЃЌЙ§ТЫГ§ШЅFeЃЈOHЃЉ 3КѓЃЌдйМгШыCaCO3ЕїНкpHЕН5.4ЃЌЙ§ТЫЕУЕНAlЃЈOHЃЉ 3???? ЃЈ2ЗжЃЉ
ЃЈ5ЃЉAlCl3БЅКЭШмвКжаДцдкШмНтЦНКтЃКAlCl3ЁЄ6H2OЃЈsЃЉ Al3+ЃЈaqЃЉ +3ClЁЊЃЈaqЃЉ +6H2OЃЈlЃЉЃЌЭЈШыHClЦјЬхЪЙШмвКжаcЃЈClЁЊЃЉдіДѓЃЌЦНКтЯђЮіГіЙЬЬхЕФЗНЯђвЦЖЏДгЖјЮіГіAlCl3ОЇЬхЁЃ??? ЃЈ2ЗжЃЉ
Al3+ЃЈaqЃЉ +3ClЁЊЃЈaqЃЉ +6H2OЃЈlЃЉЃЌЭЈШыHClЦјЬхЪЙШмвКжаcЃЈClЁЊЃЉдіДѓЃЌЦНКтЯђЮіГіЙЬЬхЕФЗНЯђвЦЖЏДгЖјЮіГіAlCl3ОЇЬхЁЃ??? ЃЈ2ЗжЃЉ
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКЃЈ1ЃЉИљОнУКэЗЪЏОдЄДІРэКѓКЌГЩЗжПЩжЊЃЌФмгыHClжївЊЗДгІЕФЮЊAl2O3ЁЂFe2O3ЃЌЦфЗДгІЕФРызг
ЗНГЬЪНЮЊAl2O3+6H+==2Al3++3H2OЁЂ Fe2O3+6H+==2Fe3++3H2O ЃЛ
ЃЈ2ЃЉгАЯьЫсНўвђЫигабЮЫсЕФХЈЖШЁЂЗДгІЮТЖШЁЂБэУцЛ§ЃЈУКэЗЪЏПХСЃДѓаЁЃЉЁЂЪЧЗёГфЗжНСАшЁЂЗДгІЪБМфЕШЃЛ
ЃЈ3ЃЉЫсНўКѓЕФШмвКГЪЫсадЃЌвЛЗНУцЪЧЪЃ
грЕФHClЃЌСэвЛЗНУцЪЧAl3++3H2O AlЃЈOHЃЉ3+3H+ЁЂFe3++3H2O
AlЃЈOHЃЉ3+3H+ЁЂFe3++3H2O FeЃЈOHЃЉ3+3H+ЕФЫЎНтГЪЫсадЃЌМгШыCaCO3КѓгыЦфH+ЗДгІЩњГЩCO2ЃЛвђAlЃЈOHЃЉ3ЪЧСНадЧтбѕЛЏЮяЃЌМШФмШмвКЫсЃЌгжФмШмгкЧПМюЃЌЯђГСЕэжа
FeЃЈOHЃЉ3+3H+ЕФЫЎНтГЪЫсадЃЌМгШыCaCO3КѓгыЦфH+ЗДгІЩњГЩCO2ЃЛвђAlЃЈOHЃЉ3ЪЧСНадЧтбѕЛЏЮяЃЌМШФмШмвКЫсЃЌгжФмШмгкЧПМюЃЌЯђГСЕэжа
МгШчМюЪБЃЌЗДгІЕФРызгЗНГЬЪНЮЊAlЃЈOHЃЉ3 +OHЁЊ=AlO2ЁЊ+2H2OЃЛ
ЃЈ4ЃЉИљОнFe3+ЁЂAl3+ГСЕэЕФpHПЩжЊЃЌЪЙFe3+ГСЕэЪБЃЌAl3+РызгВЛФмГСЕэЃЌЙЪгІНЋpHЕїжСЕН3.2ЃЌЮЊСЫЛёЕУВњЦЗAlЃЈOHЃЉ 3ЃЌдђашвЊAl3+РызгЭъШЋГСЕэЃЌЙЪНЋpHЕїжСЕН5.4ЃЌдкЕкЖўВНЕїНкpHжЎЧАЃЌгІгУЙ§ТЫЕФЗНЗЈНЋFeЃЈOHЃЉ 3Г§ШЅЃЛЃЈ5ЃЉAlCl3БЅКЭШмвКжаДцдкШмНтЦНКтЃЌЭЈШыHClЦјЬхШмгкЫЎЕчРыГіClЁЊЃЌЪЙШмвКжаcЃЈClЁЊЃЉдіДѓЃЌЦНКтЯђЮіГіЙЬЬхЕФЗНЯђвЦЖЏДгЖјЮіГіAlCl3ОЇЬхЁЃ
ПМЕуЃКПМВщЛЏбЇЙЄвеСїГЬЭМЁЃжївЊПМВщгаРызгЗНГЬЪНЁЂгАЯьЛЏбЇЗДгІЕФвђЫиЁЂдЊЫиМАЦфЛЏКЯЮяЕФаджЪЁЂЕїНкШмвКЕФpHжЕГ§дгЁЂГСЕэШмНтЦНКтЁЃ


 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъИЃНЈЪЁеФжнЪаАЫаЃИпШ§ЕкШ§ДЮСЊПМРэзлЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ФГЕиУКэЗЪЏОдЄДІРэКѓКЌSiO2ЃЈ63%ЃЉЁЂAl2O3ЃЈ25%ЃЉЁЂFe2O3ЃЈ5%ЃЉМАЩйСПИЦУОЕФЛЏКЯЮяЕШЃЌвЛжжзлКЯРћгУЙЄвеЩшМЦШчЯТЃК
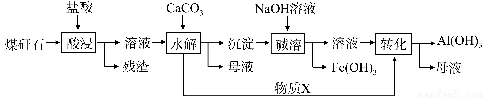
ЃЈ1ЃЉЁАЫсНўЁБЙ§ГЬжажївЊЗДгІЕФРызгЗНГЬЪНЮЊ__________?? ЁЂ____________ЁЃ
ЃЈ2ЃЉЁАЫсНўЁБЪБТСНўГіТЪЕФгАЯьвђЫиПЩФмга_____? ________ЁЂ_____? ______ЁЃЃЈаДГіСНИіЃЉ
ЃЈ3ЃЉЮяжЪXЕФЛЏбЇЪНЮЊ___________ЁЃЁАМюШмЁБЪБЗДгІЕФжївЊРызгЗНГЬЪНЮЊЃКFe3++3OH- = Fe(OH)3Ё§ЃЛ??????????????????? ????????????????? ЁЃ
ЃЈ4ЃЉвбжЊFe3+ПЊЪМГСЕэКЭГСЕэЭъШЋЕФpHЗжБ№ЮЊ2.1КЭ3.2ЃЌAl3+ПЊЪМГСЕэКЭГСЕэЭъШЋЕФpHЗжБ№ЮЊ4.1КЭ5.4ЮЊСЫЛёЕУВњЦЗAl(OHЃЉ3ЃЌДгУКэЗЪЏЕФбЮЫсНўШЁвКПЊЪМЃЌШєжЛгУCaCO3вЛжжЪдМСЃЌКѓајВйзїЙ§ГЬЪЧ?????????????????????? ЁЃ
ЃЈ5ЃЉвдУКэЗЪЏЮЊдСЯЛЙПЩвдПЊЗЂЦфЫћВњЦЗЃЌР§ШчдкУКэЗЪЏЕФбЮЫсНўШЁвКГ§ЬњКѓЃЌГЃЮТЯТЯђAlCl3ШмвКжаВЛЖЯЭЈШыHClЦјЬхЃЌПЩЮіГіДѓСПAlCl3ЁЄ6H2OОЇЬхЃЌНсКЯЛЏбЇЦНКтвЦЖЏдРэНтЪЭЮіГіОЇЬхЕФдвђЃК???????????????????????????????????????? ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com