
(8��)��һ�������¿�ʵ����ͼ��ʾ����֮��ı仯:
����д���¿հ�:
��1����ȸʯ����Ҫ�ɷ���Cu2(OH)2CO3�������ֽ⡣��ͼ�е�F�ĵ���ʽΪ ��
��2��д��������Һ�����NaOH��Һ��Ӧ�����ӷ���ʽ ��
��3��ͼ������G��D��Ϊ���壬��ͺ��ڸ����¿ɷ�����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4��D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ���õ����ű������ת�Ƶķ�����Ŀ�� ��
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

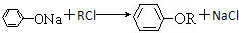








 ������Ӧ�Ļ�ѧ����ʽ�ǣ���һ����
������Ӧ�Ļ�ѧ����ʽ�ǣ���һ����



 �ṹ����
�ṹ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




| Ũ���� |
| �� |
| Ũ���� |
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

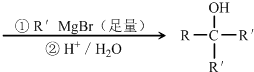




 +2NaOH
+2NaOH
 +NaBr+2H2O
+NaBr+2H2O +2NaOH
+2NaOH
 +NaBr+2H2O
+NaBr+2H2O

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |








�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
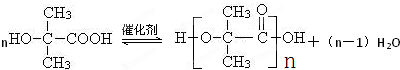
����ת����ϵ R��CH2OH![]() R��CHO
R��CHO![]() RCOOH
RCOOH
��Է������� M M��2 M+14
��֪��Aֻ��C��H��OԪ�أ���A��C��H��������֮��Ϊ56.8������һ���������ܷ���������Ӧ��A����������֮������ͼ��ʾ��ת����ϵ(MA��McΪA��C������Է�������)����ش�

(1)д���ṹ��ʽ��A________��E________��G________
(2)A��ͬ���칹���ж��֣���д������ͬ���칹��Ľṹ��ʽ��
___________________________��_____________________________��
(3)��A����F�ķ�Ӧ������________��
(4)��C��D��һ��������Ӧ�������ɻ�״������䷴Ӧ����ʽΪ_____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com