
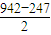 kJ/mol=347.5kJ/mol������Խ��ѧ��Խ�ȶ����Դ��жϣ�
kJ/mol=347.5kJ/mol������Խ��ѧ��Խ�ȶ����Դ��жϣ� =1����ɫ��Ϊ12×
=1����ɫ��Ϊ12× =3���ʰ�ɫ��ΪN3-����ɫ��ΪCu+����N3-Ϊ���ģ���X��Y��Z�������ϣ���ÿ�����ϵȾ����Cu+��2�������ݺ�������Ų�������дCuԭ�ӵĻ�̬�����Ų�ʽ��
=3���ʰ�ɫ��ΪN3-����ɫ��ΪCu+����N3-Ϊ���ģ���X��Y��Z�������ϣ���ÿ�����ϵȾ����Cu+��2�������ݺ�������Ų�������дCuԭ�ӵĻ�̬�����Ų�ʽ��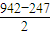 kJ/mol=347.5kJ/mol����N2�еĦм����ܴ��ڦҼ����ܣ��м����ȶ����ʴ�Ϊ���У��ң�
kJ/mol=347.5kJ/mol����N2�еĦм����ܴ��ڦҼ����ܣ��м����ȶ����ʴ�Ϊ���У��ң�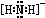 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� HD��+NH3��+HDO��
HD��+NH3��+HDO�� =1����ɫ��Ϊ12×
=1����ɫ��Ϊ12× =3���ʰ�ɫ��ΪN3-����ɫ��ΪCu+����N3-Ϊ���ģ���X��Y��Z�������ϣ���ÿ�����ϵȾ����Cu+��2�����ʹ�ͬһ��N3-������X+��6����
=3���ʰ�ɫ��ΪN3-����ɫ��ΪCu+����N3-Ϊ���ģ���X��Y��Z�������ϣ���ÿ�����ϵȾ����Cu+��2�����ʹ�ͬһ��N3-������X+��6����

 �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪�ֳ���ʵ����ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��9�֣���Ԫ�ؿ��γɶ������ӣ���N3����N3����NH2����NH4+��N2H5+��N2H62+�ȡ�
��1����N���ڵ�����Ԫ��C��O�����ߵ�һ�������ɴ�СΪ_______
��2��N��N�ļ���Ϊ942 kJ��mol-1��N��N�����ļ���Ϊ247 kJ��mol-1��˵��N2�е� ���� ���ȶ�����ҡ��С�����
��3��Һ̬���ɵ����NH2����NH2���ĵ���ʽΪ ��
��4����֪NH4HΪ���ӻ����д��������ˮD2O��Ӧ����������ȣ� ��Na3NҲΪ���ӻ������Na3N��ˮ��Ӧ�Ļ�ѧ����ʽΪ ����Ӧ����Ϊ ��
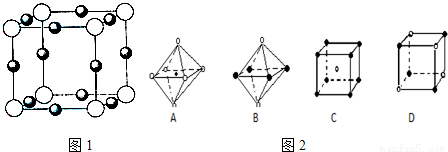
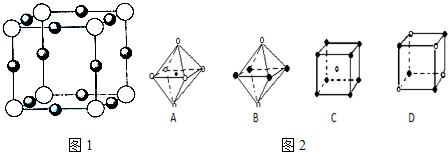
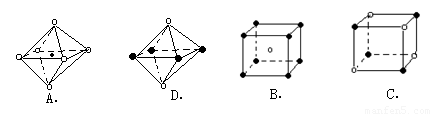
��5��X+�����е������ó���K��L��M�������Ӳ㣬����N3���γɵľ���ṹ��ͼ��ʾ����ͬһ��N3��������X+ �� ����Xԭ�ӵĻ�̬�����Ų�ʽΪ_____________
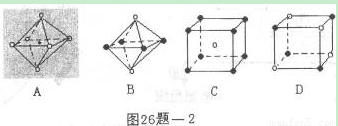
��6�������ѧ�����Ƴ�ijԪ��Z��NԪ���γɵľ���ZN����֪ZN���������NaCl���Ƶľ���ṹ����ͼ�Ǵ�ZN����ṹͼ�зָ�����IJ��ֽṹͼ�����жϷ���ZN����ṹͼ����_______
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������и���9���¿������ۺ����⣨��ѧ���֣� ���ͣ������
(14��)���ǵ����ϼ�Ϊ�ḻ��Ԫ�أ���Ԫ�ؿ��γɶ������ӣ��磺N3����N3����NH2����NH4+��N2H5+��N2H62+�ȡ�
(1)�뻭������ԭ�ӽṹʾ��ͼ___________��
(2)���ij����⻯��ĽṹʽΪ________����ռ乹��Ϊ_______������������ˮ����Ҫԭ����_________________������ˮ���Լ��Ե�ԭ����_______________(�����ӷ���ʽ˵��)
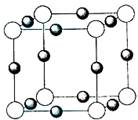
(3)X+�����е������ó���K��L�������Ӳ㣬����N3���γɵľ���ṹ��ͼ26�� l��ʾ����ͬһ��N3��������X+��________����X��Ԫ�ط�����________��
(4)�����ѧ�����Ƴ�ijԪ��Z��NԪ���γɵľ���ZN����֪ZN���������NaCI���Ƶľ���ṹ��ͼ26��-2�Ǵ�ZN����ṹͼ�зָ�����IJ��ֽṹͼ�����жϷ���ZN����ṹͼ����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0113 ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08�㽭��ɽ��ѧ�¿�)��Ԫ�ؿ��γɶ������ӣ���N3�D��N3�D��NH2�D��NH4+��N2H5+��N2H62+�ȣ�����N2H62+�������Է��ӽ�������γɵģ���������NH4+�����ʡ��ش��������⣺
��1��N3�D���Ӱ뾶��Na+���Ӱ뾶 �����С����
��2��д��Na3N��ˮ��Ӧ�Ļ�ѧ����ʽ
��3��NH4+������N�DH���ļ���Ϊ ��д�����ӻ�����NH5�ĵ���ʽ
��4��д��N2H62+���������ǿ����Һ��Ӧ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com