
�������ƣ�NaClO2����һ����Ҫ�ĺ�������������Ҫ����ˮ�������Լ�ɰ�ǡ���֬��Ư����ɱ���������ǹ������ⷨ�����������ƵĹ�������ͼ��
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2?3H2O��
�ڴ�ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10�����°�ȫ��
��160 g/L NaOH��Һ��ָ160 gNaOH��������ˮ������Һ�����Ϊ1L��
��1��160 g/L NaOH��Һ�����ʵ���Ũ��Ϊ ����Ҫ�������Һ����������������Ҫ��һ�������� ��������˵������
��2���������й�����������ÿ����� ��ѡ����ţ���
a����SO2������SO3����ǿ���ԣ� b��ϡ��ClO2�Է�ֹ��ը��
c����NaClO3������ClO2
��3���������ڵķ�Ӧ�����ӷ�Ӧ����ʽΪ ��
���������¶Ȳ��ܳ���20�棬��Ŀ���� ��
��4���ڼ�����Һ��NaClO2�Ƚ��ȶ���������������Ӧά��NaOH�Թ������ж�NaOH�Ƿ�����ļ�ʵ�鷽���� ��
��5����������Ϊ��ֹNaClO2����ԭ��NaCl�����û�ԭ���Ļ�ԭ��Ӧ���С���H2O2�⣬������ѡ��Ļ�ԭ���� ��ѡ����ţ���
a��Na2O2 b��Na2S c��FeCl2
��6������Һ�еõ�NaClO2?3H2O�־����ʵ����������� ��ѡ����ţ�
a������ b������ c������ d������ e����ȴ�ᾧ
Ҫ�õ�������NaClO2?3H2O���������еIJ����� ����������ƣ�
��1��4 mol/L ��Һ���ܶȣ�2��b
��3��2OH-+2ClO2+H2O2��2ClO2-+O2��+2H2O ��ֹH2O2���ȷֽ⣬������NaClO2?3H2O������
��4�������ⶨ����������Һ��pHֵ ��5�� a��6��bed �ؽᾧ
���������������1������Ϣ��֪��160g/LNaOH��Һ��ʾ1L����������Һ����160gNaOH���������ʵ���Ũ�ȵĶ���ʽc=n/v����ɵø���Һ�������Ƶ����ʵ���Ũ��Ϊ4mol/L���������ʵ���Ũ�Ⱥ����������Ļ��㹫ʽ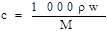 ��֪��Ҫ����������������Ҫ֪����Һ���ܶȡ�
��֪��Ҫ����������������Ҫ֪����Һ���ܶȡ�
��2������Ϣ�ڿ�֪����ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10%���°�ȫ���������й������������Ӧ��ϡ��ClO2�Է�ֹ��ը��
��3�����ݹ�������ͼ��֪���������ڵķ�Ӧ��ΪClO2��H2O2��NaOH��Һ����������NaClO2������һ����ClO2ת��ΪNaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض�����������������������Ӧ�����ӷ���ʽΪ2OH-+2ClO2+H2O2��2ClO2-+O2��+2H2O��H2O2���ȶ����¶ȹ������ֽ⣬���������¶Ȳ��ܳ���20�棬��Ŀ���Ƿ�ֹH2O2�ֽ⡣�����Ϣ��NaClO2���ܽ�����¶����߶��������������¶Ȳ��ܳ���20���ԭ��������NaClO2?3H2O��������
��4��NaOH��������Һ�ʼ��ԣ��ⶨ��Һ�����������ǣ������ⶨ����������Һ��pH��
��5����������ClO2ת��ΪNaClO2�Ļ�ԭ��ΪH2O2��Na2O2����ˮ�൱��H2O2����Na2S��FeCl2��ԭ�Խ�ǿ��ѡa��
��6������Һ�еõ����ᾧˮ�ľ����ȡ������Ũ������ȴ�ᾧ������ͨ�����˵õ��־��壮���Բ���˳��Ϊbed���õ��Ĵ־��徭���ؽᾧ�ɵõ����ȸ��ߵľ��塣
���㣺���������������Ʊ��Ļ�ѧ��������Ϊ���壬����ѧ���Ķ���Ŀ��ȡ��Ϣ�����������ʵ���Ũ�ȼ��㡢������ԭ��Ӧ���֪ʶ�ͻ�ѧ����ʵ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϳ��ú�����SiO2��Al2O3�ĸ�����FeO Cr2O3��ұ��������Ҫ�������£�
Cr2O3��ұ��������Ҫ�������£�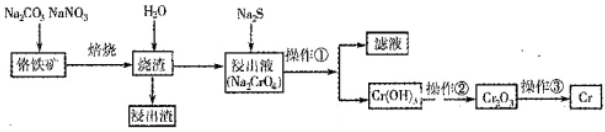
��1��������л�ѧ����ʽ���ں�����д���ʵĻ�ѧʽ��ϵ������
2FeO��Cr2O3��4Na2CO3��7NaNO3 4Na2CrO4��Fe2O3��4CO2��______________��
4Na2CrO4��Fe2O3��4CO2��______________��
��2�������ٰ���������ϴ�ӣ���ʵ�����н���ϴ�ӳ����IJ���__________________________________�������ڿ�ѡ�õ�װ�ã����ּг�װ����ȥ����________������ţ�
��3��д���ܹ���ɲ����۵���ط�Ӧ�Ļ�ѧ����ʽ��_____________________��
��4����ѧ��������COD���ɶ���ˮ�����л�����Ⱦ�ij̶ȡ���ǿ�Ტ���ȵ������£���K2Cr2O7��ǿ����������ˮ�������ⶨ���ĵ�K2Cr2O7������Ȼ������൱��O2�ĺ�����Ϊ��ѧ����������mg/L�ƣ�����ѧ��ȤС��ⶨijˮ���Ļ�ѧ��������COD���������£�
I.ȡamLˮ��������ƿ������10��00mL 0.2500 mol/L��K2Cr2O7��Һ��
II��������
III����ָʾ������c mol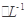 �����������[ ��NH4��2Fe��SO4��
�����������[ ��NH4��2Fe��SO4�� ]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
��I����ȡK2Cr2O7��Һ��������_____________��
�ڼ����ˮ���Ļ�ѧ������ʱ���õ����й�ϵ��Ҫ��ȥ1molCr2O72�� ������___molFe2����1molCr2O72���൱��_______ molO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�ۺ����õĹ�������ͼ���£�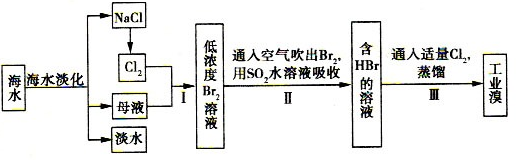
��l�����NaCl��Һ���ڵ����п�ֱ�ӵõ��IJ�Ʒ��H2 �� ��H2�� ��
��2������I���ѻ��Br2������II���ֽ�Br2��ԭΪBr-����Ŀ���� ��
��3������II��SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ ����Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������ ��
��4����������κ�ĺ�ˮ���̺��ŷḻ��þ��Դ����ת����ɻ��MgCl2�ֲ�Ʒ���Ӻ�ˮ����ȡþ�IJ���Ϊ��
a�������ߴ������ڵı��Ƕ��ճ�ʯ�ң�����ʯ���Ƴ�ʯ���飻
b����ʯ������˺�ˮ�������о����˵õ�Mg(OH)2������
c����Mg(OH)2�����м�������õ�MgCl2��Һ���پ�������Ʒ�õ�MgCl2�� 6H2O;
d����MgCl2��6H2O��һ�������¼��ȵõ���ˮMgCl2��������ڵ��Ȼ�þ�ɵõ�Mg��
�ٲ���d�еġ�һ��������ָ���� ��Ŀ���� ;
����ͬѧ��Ϊ������b��ɼ���Mg(OH)2�õ�MgO���ٵ�����ڵ�MgO�ƽ���þ�������ɼ��������裬���ּ�Լ��ԭ�����Ƿ�ͬ���ͬѧ���뷨��˵�����ɣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���и������ʵķ�����ᴿ��Ӧѡ��������������һ�֣�����ѡ����ĸ��
| A����Һ | B������ | C����ȡ | D������E�������ᾧ����F�����·ֽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ�Ƿ�������ʱ���õ��������ش���������:
��1�������C��E������ ��
��2���������»����Ӧ����Ҫѡ������ʲô������������ĸ���ţ�
��NaCl�������ɳ�� �ڻ����ͺ�ˮ��
��3�����й���������ʹ��˵����ȷ����
A��A���������þƾ���ֱ�Ӽ���
B��B��������������ƾ��������Ӿƾ�ks5u
C��C�����ڷų�Һ��ʱӦ���ϱߵ�ƿ��
D����ʵ����Ӧ��D��������ʵ��ʱҪ�����ò���������
E��������ˮʱE������ˮ���������Ͻ��³�
��4������Cװ���м����ˮ������CCl4��������ã���۲쵽ʲô����
��5���ⵥ�ʺ��嵥���������Ƶ����ʣ����������л��ܼ���ȡˮ��Һ�еĵ��ʣ�������C������ȡ��ˮ�е��嵥�ʣ������л��ܼ��в���ѡ�õ��ǣ�
A������ B��CCl4 C���ƾ� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺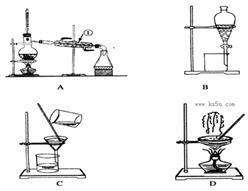
��1�����Ȼ�����Һ�еõ��Ȼ��ع��壬ѡ��װ�� (�����װ��ͼ����ĸ����ͬ)����ȥ����ˮ�е�Cl�������ʣ�ѡ��װ�� ��
��2���ӵ�ˮ�з����I2��ѡ��װ�� ���÷��뷽��������Ϊ ��
��3��װ��A�Тٵ������� ����ˮ�ķ����Ǵ� �ڽ�ˮ��װ��B�ڷ�ҺʱΪʹҺ��˳���µΣ�Ӧ���еľ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����п��ZnFe2O4���ǶԿɼ������еİ뵼���������ʵ�����Ʊ�ԭ��Ϊ��
Zn2++2Fe2++3C2O42��+6H2O ZnFe2(C2O4)3��6H2O������������(a)
ZnFe2(C2O4)3��6H2O������������(a)
ZnFe2(C2O4)3��6H2O  ZnFe2O4+2CO2��+4CO��+6H2O ����������(b)
ZnFe2O4+2CO2��+4CO��+6H2O ����������(b)
��1�������Ʊ�ԭ��������������ԭ��Ӧ���� ��ѡ���a����b������
��2���Ʊ�ZnFe2(C2O4)3��6H2Oʱ����ѡ�õ�ҩƷ�У�
��(NH4)2Fe(SO4)2��6H2O����ZnSO4��7H2O����(NH4)2C2O4��7H2O��
�ٳ���ҩƷʱ�������ϸ����n(Fe2+)/n(Zn2+)= ��
��ѡ�õļ��Ϸ�ʽ�� (����ĸ)��
a����һ�������ȣ���ͬʱ���뷴Ӧ������ˮ���裬Ȼ��������75�档
b����һ�������ȣ���ͬʱ���뷴Ӧ����ˮ�����Һ��Ȼ������Ȼ�����µ�75�档
c����һ�������ȣ������ϲ������Һ�ף��������������Һ�ң��ס���ͬʱ���ȵ�75�棬Ȼ������Һ�����������Һ�У����������衣
��3������Һ�з����ZnFe2(C2O4)3��6H2O����ˡ�ϴ�ӡ���ϴ����ȫ�������� ��
��4��ZnFe2(C2O4)3��6H2O�ȷֽ����þƾ���ƣ����õ��Ĺ������������� �� ��
��5��ij��ѧ����С�����÷Ͼɸɵ��пƤ�������������������ͼ��Ϣ����ʵ����ṩ���Լ���ȡ������ZnSO4��Һ��
ʵ���п�ѡ�õ��Լ����£�
��a��30%H2O2����b��������ˮ��
��c��1.0 mol��L��1NaOH��Һ��
��d��3mol��L-1ϡ���
��e����ZnO��ĩ��
��f����п�ۡ�
ʵ�鲽������Ϊ���ٽ�пƬ��ȫ�����Թ�����3mol��L��1ϡ���ᣬ���� ��ѡ����ĸ����ͬ�����ڼ��� ���ۼ��ȵ�60�����Ҳ����Ͻ��裻
�ܹ��˵�ZnSO4��Һ�����в���ۼ��ȵ���ҪĿ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȥ���������������������ʣ���д��ѡ�õ��Լ��ͷ��뷽��
| | ����� (������Ϊ��������) | �Լ� �������� | ���뷽�� |
| A | �������ӣ� | | |
| B | ��ϩ��SO2�� | | |
| C | �������������ᣩ | | |
| D | �Ҵ���ˮ�� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��NH4+��Mg2+��Al3+��SO42����NO3����Cl�� ��ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ������������Һ����Ʋ���������µ�ʵ�飺
��֪��3NO3��+ 8Al + 5OH�� + 2H2O 3NH3 + 8AlO2��
3NH3 + 8AlO2��
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ����
�����п϶�����NH4+��Mg2+��SO42����NO3��
������һ������Al3+
�����п��ܴ���Na+��Cl��
�������п��ܴ���NaNO3��NH4Cl��MgSO4
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com