
����Ŀ����ҵ�ϳ��ø�Ĥ��ⷨ����ȩת��Ϊ�Ҵ���������������Ũ����ȩ��ˮ��̽����ѧϰС������ͼ��ʾװ�õ��һ��Ũ�ȵ���ȩ-Na2SO4��Һ��ģ����ȩ��ˮ�Ĵ������̡�����˵����ȷ����
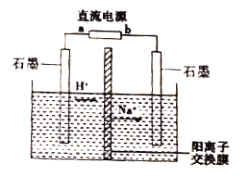
A. a Ϊֱ����Դ�ĸ���
B. �����ĵ缫��ӦΪ:CH3CHO-2e-+H2O=CH3COOH+2H+
C. �������У���������pH��С
D. ������������������ˮ��������������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������һ�ֳ��õ�ҽҩ�м��壬�ϳ�·�����£�

��1���ٵķ�Ӧ������_________���ڵķ�Ӧ�Լ���������________________��
��2����Ӧ����������Ӧ������һ���ǻ�û�б�������ԭ����___________________��
�����鷴Ӧ�ܵ��л������ѡ����Լ���________________________��
a. Na b. NaCl��Һ c. NaOH��Һ d. NaHCO3��Һ
��3��д����Ӧ�ݵĻ�ѧ����ʽ__________________________________��д��һ���밢���������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ__________________��
��4����CH2=CH-CHO���Ҵ����Ժϳ�CH3CH2COOC2H5��д����ϳ�·�ߡ�____________
���ϳ�·�߳��õı�ʾ��ʽΪ��X![]() Y����
Y����![]() Ŀ�������
Ŀ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�������ȷ�Ӧ����( )
A. C6H12O6(������aq)��6O2![]() 6CO2��6H2O
6CO2��6H2O
B. ����������Һ��������кͷ�Ӧ
C. ��Ӧ��������������������������
D. �ƻ���Ӧ��ȫ����ѧ���������������ƻ�������ȫ����ѧ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(��)�ǰ��Ƶ��в�ҩ��ҩЧ�ɷ֣�Ҳ���ö��AΪԭ���Ʊ����ϳ�·��������

�ش�����������
��1�����ķ�Ӧ����Ϊ_____________________��B�����������_________��ԭ�ӹ�ƽ�档
��2��C�к��������ŵ�����Ϊ______________________�����ġ�����a��Ϊ____________________��
��3����Ϊ�ӳɷ�Ӧ����ѧ����ʽΪ__________________________________��
��4�����Ļ�ѧ����ʽΪ__________________________________��
��5�����㻯����J��D��ͬ���칹������������������J�Ľṹ����_________�������к˴Ź�������Ϊ������J�Ľṹ��ʽΪ_________________��(ֻдһ�ּ���)��
��������ֻ��3��ȡ������������NaHCO3��Ӧ�ų�CO2����1mol J���к�3mol NaOH��
��6��������ͼ��Ϣ��д���� Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·��(���Լ���ѡ) ��______________
�ĺϳ�·��(���Լ���ѡ) ��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.2Al+Fe2O3![]() Al2O3+2Fe�ڻ�ѧ��Ӧ�����в��������ȣ������ڸֹ캸�ӡ����������գ�
Al2O3+2Fe�ڻ�ѧ��Ӧ�����в��������ȣ������ڸֹ캸�ӡ����������գ�
��1����Ԫ����Ԫ�����ڱ��е�λ��Ϊ_______________�������ӽṹʾ��ͼΪ__________��
��2�����γ�������ˮ���������ֺͻ�ѧ������н���_______________________________��
��3�����ķǽ�����ǿ�ڵ�����Ԫ��������֪ʶ����ԭ��_____________________________��
��4��Al2O3��NaOH��Һ��Ӧ�����ӷ���ʽΪ______________________________________��
II.NH4Al(SO4)2��12H2Oˮ��Һ��_____�ԣ�ѡ��ᡱ��������С������Ƚ���Һ������Ũ�ȴ�Сc(NH4+)_____c(Al3+)��ѡ���������������=������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.�ǽ���ԭ�Ӽ䲻�����γ����Ӽ���
B.��ͬԭ���γɵĻ�ѧ��һ���Ǽ��Լ���
C.���ۻ������в����ܺ����Ӽ���
D.���ӻ������в����ܺ��й��ۼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2A+B3C+4D��Ӧ�У���ʾ�÷�Ӧ���������ǣ�������
A.v��A��=0.5 mol/��Ls��
B.v��B��=0.3 mol/��Ls��
C.v��C��=0.8 mol/��Ls��
D.v��D��=1 mol/��Ls��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��18��Ԫ�ؼ����γɵĻ�����Ļ�ѧʽ��ա�
��1��ԭ�Ӱ뾶��С��Ԫ����________��
��2����ϡ������Ԫ���⣬ԭ�Ӱ뾶����Ԫ����________������ԭ�ӽṹʾ��ͼ��________��
��3����ˮ��Ӧ����ҵĽ�����________��
��4������������Ӧ��ˮ���������ǿ����________��
��5������������Ӧ��ˮ����Ϊ���������������________��
��6����̬�⻯���ˮ��Һ�ʼ��Ե�Ԫ����________��
��7�����ȶ�����̬�⻯����________��
��8����������ǿ��Ԫ����________���ǽ�������ǿ��Ԫ����________��
��9������������Ӧ��ˮ����������ǿ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£��ֱ�ȡδ֪Ũ�ȵ�MOH��HA��Һ����ˮϡ����ԭ�����n����ϡ�����У�����ҺpH�ı仯����ͼ��ʾ������������ȷ����

A. MOHΪ���HAΪǿ��
B. ˮ�ĵ���̶ȣ�X=Z>Y
C. �������¶ȣ�Y��Z���Ӧ��Һ��pH������
D. ��X����Һ��Z����Һ�������ϣ�������Һ�ʼ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com