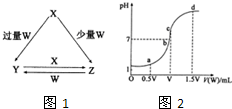
��2013?�����ģ��X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ1��ʾ��ˮ�����ֲ�������ȥ����
��1����XΪ�������ʣ�W��ijǿ���ϡ��Һ��X������W��Ӧ����Z�����ӷ���ʽΪ
3Fe+8H++2NO3-=3Fe2++2NO��+4H2O
3Fe+8H++2NO3-=3Fe2++2NO��+4H2O
����Y��Һ�м���ij���Լ�
���軯��
���軯��
�����Լ����ƣ�������Һ����Ѫ��ɫ�������ж�Y��Һ�������ӵĴ��ڣ�
��2����X��YΪ���Σ�X��ˮ��Һ�����ԣ�WΪNaOH��Һ��д��Y��X��ˮ��Һ��ת��ΪZ�����ӷ�Ӧ����ʽ
Al3++3AlO2-+6H2O=4Al��OH��3��
Al3++3AlO2-+6H2O=4Al��OH��3��
��
��3����XΪǿ�������WΪ�д̼�����ζ����̬���������ʱ����Z��ˮ��Һ¶���ڿ����У���Һ��PH�仯��
��С
��С
����������С���������䡱��������ˮ�Ļӷ�������ԭ����
������������л�ԭ�ԣ��ױ������е���������������������ӣ�2SO32-+O2=2SO42-��������Һ��pH��С
������������л�ԭ�ԣ��ױ������е���������������������ӣ�2SO32-+O2=2SO42-��������Һ��pH��С
���ü�Ҫ������˵������д�����ӷ���ʽ��
��4�������£�����0.1mol/L��NaOH��Һ�ζ�VmL0.1mol/L HA��Һ���ζ�������2ͼ��ʾ����a��b��c��d�ĵ���Һ��ˮ�ĵ���̶�������
c
c
�㣻a����Һ������Ũ�ȵĴ�С˳��Ϊ
c��A-����c��Na+����c��H+����c��OH-��
c��A-����c��Na+����c��H+����c��OH-��
��ȡ����c����Һ���Թ��У��ٵμ�0.1mol/L���������ԣ���ʱ��Һ�г�H
+��OH
-�⣬����Ũ�ȵĴ�С˳��Ϊ
c��Na+����c��A-����c��Cl-��
c��Na+����c��A-����c��Cl-��
��

 ��2013?�����ģ��[��ѧ-���ʽṹ������]
��2013?�����ģ��[��ѧ-���ʽṹ������]

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�
 ��2013?�����ģ��X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ1��ʾ��ˮ�����ֲ�������ȥ����
��2013?�����ģ��X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ1��ʾ��ˮ�����ֲ�������ȥ���� 