
 ʵ�����Ʊ������鲢���������������ʵ�����£��Իش��������⣺
ʵ�����Ʊ������鲢���������������ʵ�����£��Իش��������⣺���� I����1��Ũ������廯�Ʒ�Ӧ����HBr���Ҵ���HBr����ȡ����Ӧ���������飻
��2��Ũ���Ὣ���ɵ��廯�����������嵥�ʣ�������������ԭ��֪�����������������ж�ʹ��������ػ�ɫ�������ǿ�����ԣ���������ԭ�����ʣ���ȥ������������ʱ���������µ����ʣ�
��3��Ҫ�����������е���Ԫ�أ�Ӧ���Ƚ��������е���ԭ��ת��Ϊ�����ƶ��������ӣ�Ȼ��������������Һ���������ӣ�
II����4���ڼ��������£���������������ƵĴ���Һ������ȥ��Ӧ������ϩ��HBr��HBr���Ҵ���������ˮ����ϩ��������ˮ����ϩ�ܱ����Ը��������Һ������ʹ���Ը��������Һ��ɫ��
��� �⣺��1��Ũ������廯�Ʒ�Ӧ����HBr���Ҵ���HBr����ȡ����Ӧ���������飬����������ķ�Ӧ����ʽΪC2H5OH+HBr$\stackrel{��}{��}$C2H5Br+H2O��
�ʴ�Ϊ��C2H5OH+HBr$\stackrel{��}{��}$C2H5Br+H2O��
��2��Ũ���Ὣ���ɵ��廯�����������嵥�ʣ�������������ԭ��֪�����������������ж�ʹ��������ػ�ɫ�������ǿ�����ԣ���������ԭ�����ʣ���ȥ������������ʱ���������µ����ʣ�
A��NaOH��Һ�ܴٽ�������ˮ�⣬�ʲ�ѡ��
B������H2O���ܽ�Ȳ��ʲ�ѡ��
C��Na2SO3��Һ���л�ԭ�ԣ��ܱ����������Һ������鲻��Ӧ����ѡ��
D��CCl4���ܽ���������壬�ʲ�ѡ��
��ѡC��
��3��Ҫ�����������е���Ԫ�أ�Ӧ���Ƚ��������е���ԭ��ת��Ϊ�����ƶ��������ӣ�Ȼ��������������Һ���������ӣ���������鷽���ǣ�ȡ������Һ���Թ��У�Ȼ�����Թ��м�������NaOH��Һ�����ȣ���ȴ������Һ�м���ϡ���ᣬ��������������Һ�������Ƿ��������ɫ�����ж���Ԫ�أ�
��������������Ǣܢ٢ݢۢڣ�
�ʴ�Ϊ���ܢ٢ݢۢڣ�
��4���ڼ��������£���������������ƵĴ���Һ������ȥ��Ӧ������ϩ��HBr��HBr���Ҵ���������ˮ���Ҵ���HBr���ܱ����Ը��������Һ������ʹ���Ը��������Һ��ɫ����ϩ��������ˮ����ϩ�ܱ����Ը��������Һ������ʹ���Ը��������Һ��ɫ��
���Կ����������ǣ��ұ��Թ������Ը��������Һ��ɫ������Թ���ˮ�������dz�ȥ�Ҵ���զ���ĸ��ţ�
�ʴ�Ϊ����ʹ���Ը��������Һ��ɫ����ȥ�Ҵ������ʵȸ��ţ�
���� ���⿼�������Ʊ�ʵ�飬Ϊ��Ƶ���㣬��ȷʵ��ԭ�����������������ǽⱾ��ؼ���֪��±������±Ԫ�ؼ��鷽����ʵ�������״����ǣ�5�����Ҵ��ܱ����Ը��������Һ��������Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na��Mg��AlԪ������ϼ��������� | B�� | P��S��Clԭ�Ӱ뾶���μ�С | ||
| C�� | N��O��FԪ�طǽ��������μ��� | D�� | Li��Na��Kԭ�ӵĵ��Ӳ����������� |
�鿴�𰸺ͽ���>>
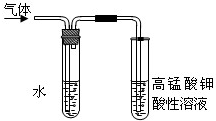
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ʵ��ǰ | ʵ��� |
| ҩƷ+U�ιܵ�����/g | 101.1 | 102.9 |
| ҩƷ+���ƿD������/g | 312.0 | 314.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��״ | �۵�/�� | �е�/�� | ˮ���� | |
| N2H4 | ��ɫҺ�� | 1.4 | 113 | ��ˮ���� |
| N2H6SO4 | ��ɫ���� | 254 | / | ������ˮ����������ˮ |
| ���� | ���� | ���� |
| �� | ȡ10g NaClO���壬����100mLˮ | �����ܽ⣬��Һ�ʼ��� |
| �� | 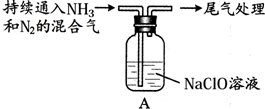 | Һ���Ϸ����ְ��� |
�鿴�𰸺ͽ���>>
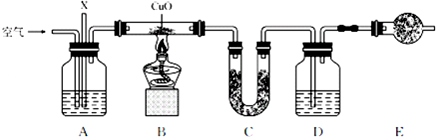
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ɷ� | Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫK2FeO4��KNO2�Ȳ��� |
| ʪ�� | ǿ���Խ����У�Fe��NO3��3��NaClO��Ӧ�����Ϻ�ɫNa2FeO4��Һ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
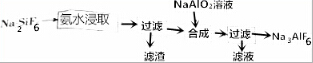
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�22.4L��NO2��CO2������庬��2NA��Oԭ�� | |
| B�� | ���³�ѹ�£�18gH2O�к��е�ԭ������Ϊ3NA | |
| C�� | ��״���£�11.2LCH3CH2OH�к��еķ�����ĿΪ0.5NA | |
| D�� | ���³�ѹ�£�2.24LCO��CO2��������к��е�̼ԭ����ĿΪ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com