
°æƒø°ø‘⁄1200 °Ê ±£¨ÃÏ»ª∆¯Õ—¡Ú𧓒÷–ª·∑¢…˙œ¬¡–∑¥”¶£∫H2S(g)£´![]() O2(g)= SO2(g)£´H2O(g)°°¶§H1£¨H2S(g)£´
O2(g)= SO2(g)£´H2O(g)°°¶§H1£¨H2S(g)£´![]() O2(g)= S(g)£´H2O(g)°°¶§H2£¨2H2S(g)£´SO2(g)=
O2(g)= S(g)£´H2O(g)°°¶§H2£¨2H2S(g)£´SO2(g)= ![]() S2(g)£´2H2O(g)°°¶§H3£¨2S(g)= S2(g)°°¶§H4 £¨‘Ú¶§H4µƒ’˝»∑±Ì¥Ô ΩŒ™£® £©
S2(g)£´2H2O(g)°°¶§H3£¨2S(g)= S2(g)°°¶§H4 £¨‘Ú¶§H4µƒ’˝»∑±Ì¥Ô ΩŒ™£® £©
A.¶§H4£Ω![]() (¶§H1£´¶§H3£≠3¶§H2)B.¶§H4£Ω
(¶§H1£´¶§H3£≠3¶§H2)B.¶§H4£Ω![]() (3¶§H2£≠¶§H1£≠¶§H3)
(3¶§H2£≠¶§H1£≠¶§H3)
C.¶§H4£Ω![]() (¶§H1£´¶§H3£≠3¶§H2)D.¶§H4£Ω
(¶§H1£´¶§H3£≠3¶§H2)D.¶§H4£Ω![]() (¶§H1£≠¶§H3£≠3¶§H2)
(¶§H1£≠¶§H3£≠3¶§H2)
 º∆À„∏fl ÷œµ¡–¥∞∏
º∆À„∏fl ÷œµ¡–¥∞∏
| ƒÍº∂ | ∏fl÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏fl“ª | ∏fl“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏fl∂˛ | ∏fl∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏fl»˝ | ∏fl»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øX°¢Y°¢Z°¢R°¢W «5÷÷∂Ã÷‹∆⁄‘™Àÿ£¨‘≠◊”–Ú ˝“¿¥Œ‘ˆ¥Û£ªÀ¸√«ø…◊È≥…¿Î◊”ªØ∫œŒÔZ2Y∫Õπ≤º€ªØ∫œŒÔRY3°¢XW4£ª“—÷™Y°¢RÕ¨÷˜◊£¨Z°¢R°¢WÕ¨÷‹∆⁄°£œ¬¡–Àµ∑®’˝»∑µƒ «
A. ºÚµ•¿Î◊”∞Îæ∂R>W>Y>Z
B. ∆¯Ã¨«‚ªØŒÔŒ»∂®–‘£∫HmW<HnR
C. Z2Y2µÁ◊” Ωø…±Ì 挙![]()
D. RY2∫ÕW2æ˘”–∆Ø∞◊–‘£¨Ω´¡Ω’flµ»ŒÔ÷ µƒ¡øªÏ∫œ»‹”⁄ÀÆ£¨∆Ø∞◊–‘‘ˆ«ø
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬¡–Àµ∑®’˝»∑µƒ «![]()
A.![]() ”–ª˙ŒÔ
”–ª˙ŒÔ![]() µƒ∑÷◊”Ω·ππ÷–∫¨”–µƒ
µƒ∑÷◊”Ω·ππ÷–∫¨”–µƒ![]() º¸ ˝ƒø“ª∂®Œ™
º¸ ˝ƒø“ª∂®Œ™![]()
B.“ª∂®Œ¬∂»œ¬£¨¬»ªØÔßÀÆΩ‚¿Î◊”∑Ω≥Ã Ω£∫![]() £¨»Ù”√
£¨»Ù”√![]() ±Ì 浃¿Î◊”ª˝£¨
±Ì 浃¿Î◊”ª˝£¨![]() ±Ì æ∞±ÀƵÁ¿Î≥£ ˝£¨‘Ú¬»ªØÔßÀÆΩ‚∆Ω∫‚≥£ ˝
±Ì æ∞±ÀƵÁ¿Î≥£ ˝£¨‘Ú¬»ªØÔßÀÆΩ‚∆Ω∫‚≥£ ˝![]()
C.“—÷™∑¥”¶£∫![]() £∫
£∫![]() £∫
£∫![]() £ª‘Ú‘⁄À·–‘»‹“∫÷–—ıªØ–‘£∫
£ª‘Ú‘⁄À·–‘»‹“∫÷–—ıªØ–‘£∫![]()
D.“—÷™
π≤º€º¸ |
|
|
|
|
º¸ƒ‹ | 360 | 436 | 431 | 176 |
‘Ú∑¥”¶![]() µƒÏ ±‰Œ™£∫
µƒÏ ±‰Œ™£∫![]()
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬¡– «¥”∫£‘ª“∫Õ÷«¿˚œı ØøÛ≤„÷–÷»°µ‚µƒ÷˜“™∑¥”¶£∫¢Ÿ2NaI£´MnO2£´3H2SO4===2NaHSO4£´MnSO4£´2H2O£´I2 ;¢⁄2NaIO3£´5NaHSO3===2Na2SO4£´3NaHSO4£´H2O£´I2œ¬¡–Àµ∑®’˝»∑µƒ «(°°°°)
A. —ıªØ–‘£∫MnO2>SO![]() >IO
>IO![]() >I2
>I2
B. I2‘⁄∑¥”¶¢Ÿ÷– «ªπ‘≠≤˙ŒÔ£¨‘⁄∑¥”¶¢⁄÷– «—ıªØ≤˙ŒÔ
C. ∑¥”¶¢Ÿ¢⁄÷–…˙≥…µ»¡øµƒI2 ±◊™“∆µÁ◊” ˝÷Ʊ»Œ™1°√5
D. NaHSO3»‹“∫≥ À·–‘£¨NaHSO3»‹“∫÷–c(HSO![]() )>c(H2SO3)>c(SO
)>c(H2SO3)>c(SO![]() )
)
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø“—÷™º◊ª˘±˚œ©À·ÀıÀÆ∏ ”Õı•(GMA)÷˜“™”√”⁄∏fl∑÷◊”Ω∫ƒ“°¢¿Î◊”Ωªªª ˜÷¨∫Õ”°À¢”Õƒ´µƒ’≥∫œº¡°£∆‰∫œ≥…¬∑œfl»Áœ¬£∫

«Îªÿ¥œ¬¡–Œ £∫
(1)GMA÷–µƒπŸƒ‹Õ≈√˚≥∆ «_________________________________°£
(2)∏ ”ÕµƒΩ·ππºÚ Ω «___________£¨FµƒΩ·ππºÚ Ω___________°£
(3)«Î–¥≥ˆB°˙C°¢D°˙EµƒªØ—ß∑Ω≥Ã Ω£¨≤¢≈–∂œ∑¥”¶¿‡–Õ£∫
B°˙C£∫_________________________________°¢___________∑¥”¶£ª
D°˙E£∫_________________________________°¢___________∑¥”¶°£
(4)Eµƒ∫À¥≈π≤’Ò«‚∆◊∑Â√ʪ˝÷Ʊ»”…¥ÛµΩ–°Œ™___________£¨EµƒÕ¨∑÷“ÏππÃÂ÷–ƒ‹∑¢…˙ÀÆΩ‚∑¥”¶µƒ¡¥◊¥ªØ∫œŒÔ”–___________÷÷°£
(5)“—÷™CH2=CH2ƒ‹‘⁄Ag◊˜¥flªØº¡µƒÃıº˛œ¬”ÎO2∑¥”¶…˙≥…![]() £¨æ›¥À«Î…˺∆“ªÃı”…±˚œ©…˙≥…Fµƒ¬∑œfl°£____________________________________________°£
£¨æ›¥À«Î…˺∆“ªÃı”…±˚œ©…˙≥…Fµƒ¬∑œfl°£____________________________________________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øŒ™¡À—–æøªØ—ß∑¥”¶A+B=C+Dµƒƒ‹¡ø±‰ªØ«Èøˆ£¨ƒ≥Õ¨—߅˺∆¡À»ÁÕºÀ˘ æ◊∞÷√°£µ±œÚ ¢”–Aµƒ ‘π‹÷–µŒº” ‘º¡B ±£¨ø¥µΩU–Œπ‹÷–º◊¥¶“∫√Êœ¬Ωµ““¥¶“∫√Ê…œ…˝°£ ‘ªÿ¥œ¬¡–Œ £∫

(1)∏√∑¥”¶Œ™________∑¥”¶(ÃÓ°∞∑≈»»°±ªÚ°∞Œ¸»»°±)°£
(2)A∫ÕBµƒ◊‹ƒ‹¡ø±»C∫ÕDµƒ◊‹ƒ‹¡ø_________(ÃÓ°∞∏fl°±ªÚ°∞µÕ°±)°£
(3)∏√∑¥”¶µƒŒÔ÷ ÷–µƒªØ—߃‹Õ®π˝ªØ—ß∑¥”¶◊™ªØ≥…________ Õ∑≈≥ˆ¿¥°£
(4)∏√∑¥”¶µƒ∑¥”¶ŒÔªØ—ߺ¸∂œ¡—Œ¸ ’µƒƒ‹¡ø________(ÃÓ°∞∏fl°±ªÚ°∞µÕ°±)”⁄…˙≥…ŒÔªØ—ߺ¸–Œ≥…∑≈≥ˆµƒƒ‹¡ø°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø“—÷™![]() «∂˛‘™»ıÀ·£¨ “Œ¬œ¬œÚƒ≥≈®∂»µƒ≤›À·»‹“∫÷–÷µŒº”»ÎKOH»‹“∫£¨À˘µ√»‹“∫÷–
«∂˛‘™»ıÀ·£¨ “Œ¬œ¬œÚƒ≥≈®∂»µƒ≤›À·»‹“∫÷–÷µŒº”»ÎKOH»‹“∫£¨À˘µ√»‹“∫÷–![]() °¢
°¢![]() °¢
°¢![]() µƒ◊È≥…∞Ÿ∑÷¬ ”ÎpHµƒπÿœµ»ÁÕºÀ˘ æ£∫œ¬¡–Àµ∑®’˝»∑µƒ «
µƒ◊È≥…∞Ÿ∑÷¬ ”ÎpHµƒπÿœµ»ÁÕºÀ˘ æ£∫œ¬¡–Àµ∑®’˝»∑µƒ «![]()
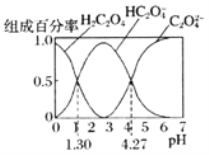
A.![]() µƒ»‹“∫÷–£∫
µƒ»‹“∫÷–£∫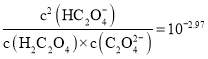
B.![]() µƒ»‹“∫÷–£¨
µƒ»‹“∫÷–£¨![]()
C.∑¥”¶![]() µƒ∆Ω∫‚≥£ ˝Œ™
µƒ∆Ω∫‚≥£ ˝Œ™![]()
D.œÚ»‹“∫÷–º”KOH»‹“∫Ω´pH”…![]() ‘ˆ¥Û÷¡
‘ˆ¥Û÷¡![]() µƒπ˝≥Ã÷–ÀƵƒµÁ¿Î≥Ã∂»œ»‘ˆ¥Û∫Ûºı–°
µƒπ˝≥Ã÷–ÀƵƒµÁ¿Î≥Ã∂»œ»‘ˆ¥Û∫Ûºı–°
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øº◊¥º «÷ÿ“™µƒªØ—ß𧓵ª˘¥°‘≠¡œ∫Õ«ÂΩ‡“∫ûº¡œ°£“—÷™÷∆±∏º◊¥ºµƒ”–πÿªØ—ß∑¥”¶“‘º∞‘⁄≤ªÕ¨Œ¬∂»œ¬µƒªØ—ß∑¥”¶∆Ω∫‚≥£ ˝»Áœ¬±ÌÀ˘ æ£∫
ªØ—ß∑¥”¶ | ∆Ω∫‚≥£ ˝ | Œ¬∂»/°Ê | |
500 | 800 | ||
¢Ÿ2H2(g)£´CO(g) | K1 | 2.5 | 0.15 |
¢⁄H2(g)£´CO2(g) | K2 | 1.0 | 2.50 |
¢€3H2(g)£´CO2(g) | K3 | ||
£®1£©æ›∑¥”¶¢Ÿ”΢⁄ø…Õ∆µº≥ˆK1°¢K2”ÎK3÷ƺ‰µƒπÿœµ£¨‘ÚK3£Ω______(”√K1°¢K2±Ì æ)°£
£®2£©∑¥”¶¢€µƒ¶§H____0(ÃÓ°∞£æ°±ªÚ°∞£º°±)°£
£®3£©500°Ê ±≤‚µ√∑¥”¶¢€‘⁄ƒ≥ ±øÃH2(g)°¢CO2(g)°¢CH3OH(g)°¢H2O(g)µƒ≈®∂»œ‡µ»£¨«“æ˘Œ™0.1mol°§L£≠1£¨‘Ú¥À ±¶‘’˝____¶‘ƒÊ(ÃÓ°∞>°±°¢°∞£Ω°±ªÚ°∞<°±)
£®4£©ƒ≥Œ¬∂»œ¬‘⁄2L∫„»›√‹±’»›∆˜÷–º”»ÎCH3OH∑¢…˙∑¥”¶2CH3OH£®g£©![]() CH3OCH3£®g£©+H2O£®g£©£¨≤‚µ√”–πÿ ˝æ›»Áœ¬£∫
CH3OCH3£®g£©+H2O£®g£©£¨≤‚µ√”–πÿ ˝æ›»Áœ¬£∫
∑¥”¶ ±º‰/min | 0 | 1 | 2 | 3 | 4 |
n£®CH3OH£©/mol | 1.02 | 0.42 | 0.22 | 0.02 | 0.02 |
¢Ÿ∑¥”¶‘⁄2minƒ⁄“‘CH3OCH3±Ì 浃ªØ—ß∑¥”¶ÀŸ¬ Œ™____£¨
¢⁄∏√Œ¬∂»œ¬µƒ∑¥”¶µƒ∆Ω∫‚≥£ ˝Œ™____°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏fl÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø¿˚”√º◊ÕȔά»∆¯∑¢…˙»°¥˙∑¥”¶÷∆»°∏±≤˙∆∑—ŒÀ·µƒ…˜Α⁄𧓵…œ“—≥…Œ™œ÷ µ£Æƒ≥ªØ—ß–À»§–°◊ÈÕ®π˝‘⁄ µ—È “÷–ƒ£ƒ‚…œ ˆπ˝≥ã¨∆‰…˺∆µƒƒ£ƒ‚◊∞÷√»Áœ¬£∫

(1)![]() ◊∞÷√”–»˝÷÷𶃋£∫¢Ÿøÿ÷∆∆¯¡˜ÀŸ∂»£ª¢⁄æ˘‘»ªÏ∫œ∆¯Ã£ª¢€________________________________________ °£
◊∞÷√”–»˝÷÷𶃋£∫¢Ÿøÿ÷∆∆¯¡˜ÀŸ∂»£ª¢⁄æ˘‘»ªÏ∫œ∆¯Ã£ª¢€________________________________________ °£
(2)…Ë![]() £¨»Ù¿Ì¬€…œ”˚ªÒµ√◊Ó∂‡µƒ¬»ªØ«‚£¨‘Ú
£¨»Ù¿Ì¬€…œ”˚ªÒµ√◊Ó∂‡µƒ¬»ªØ«‚£¨‘Ú![]() ____________________ °£
____________________ °£
(3)![]() ◊∞÷√µƒ Ø√fi÷–æ˘‘»ªÏ”–
◊∞÷√µƒ Ø√fi÷–æ˘‘»ªÏ”–![]() ∑€ƒ©£¨∆‰◊˜”√ «________________________________________________________________°£
∑€ƒ©£¨∆‰◊˜”√ «________________________________________________________________°£
(4)–¥≥ˆ![]() ◊∞÷√÷–CH4”ÎCl2…˙≥…“ª¬»¥˙ŒÔµƒªØ—ß∑¥”¶∑Ω≥Ã Ω£∫__________________________________.°£
◊∞÷√÷–CH4”ÎCl2…˙≥…“ª¬»¥˙ŒÔµƒªØ—ß∑¥”¶∑Ω≥Ã Ω£∫__________________________________.°£
(5)¢Ÿ![]() ◊∞÷√÷–≥˝—ŒÀ·Õ‚£¨ªπ∫¨”–”–ª˙ŒÔ£¨¥”
◊∞÷√÷–≥˝—ŒÀ·Õ‚£¨ªπ∫¨”–”–ª˙ŒÔ£¨¥”![]() ÷–∑÷¿Î≥ˆ—ŒÀ·µƒ◊Óº—∑Ω∑®Œ™______°£
÷–∑÷¿Î≥ˆ—ŒÀ·µƒ◊Óº—∑Ω∑®Œ™______°£
![]() °¢∑÷“∫∑®
°¢∑÷“∫∑®![]() °¢’Ù¡Û∑®
°¢’Ù¡Û∑®![]() °¢›Õ»°∑÷“∫∑®
°¢›Õ»°∑÷“∫∑®![]() °¢Ω·æß∑®
°¢Ω·æß∑®
¢⁄∏√◊∞÷√ªπ”–»±œ›£¨‘≠“Ú «√ª”–Ω¯––Œ≤∆¯¥¶¿Ì£¨∆‰Œ≤∆¯÷˜“™≥…∑÷Œ™_______°£
![]() °¢
°¢![]()
![]() °¢
°¢![]()
![]() °¢
°¢![]()
![]() °¢
°¢![]()
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
π˙º —ß–£”≈—° - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒfi÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com