
����Ŀ����������ʮ�����ʣ������ǡ�������NaCl����ʯī����ͭ˿����NaOH���塡��SO2����NaHSO4���塡��K2O���塡��Һ̬H2SO4���ⱥ��FeCl3��Һ��
��1�����������пɵ������_____(����ţ���ͬ)��
��2�����������в��ܵ��磬�����ڵ���ʵ���_____��
��3������ˮ��Һ�еĵ��뷽��ʽΪ__________________________________��
��4��ʵ�����Ʊ�����Fe(OH)3�������õ�������______(�����)������ˮ����Ӧ�����ӷ���ʽΪ_________________________________��
���𰸡��ڢۢܢ� �ݢߢ�� NaHSO4��Na++H++SO42- �� Fe3����3H2O ![]() Fe(OH)3(����)��3H��
Fe(OH)3(����)��3H��
��������
�����Ƿǵ���ʣ������磻������NaCl���������ƶ������ӣ��ܵ��磻��ʯī�������ƶ��ĵ����ܵ��磬�ǵ��ʣ���ͭ˿�к��������ƶ��ĵ��ӣ��ܵ��磬�ǵ��ʣ���NaOH���岻���磬����ˮ��Һ�ܵ��磬�ǵ���ʣ���SO2�Ƿǵ���ʣ������磻�� NaHSO4���岻���磬���ǵ���ʣ���K2O���岻���磬���ǵ���ʣ���Һ̬H2SO4�������磬���ǵ���ʣ�
������FeCl3��Һ�ܵ��磬�ǻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
(1)���������������ܵ�����Ǣڢۢܢ⣬���ڵ���ʵ��Ǣݢߢ�
��Ϊ���ڢۢܢ⣻
(2)���ݷ��������������в��ܵ��磬�����ڵ���ʵ��Ǣݢߢ�
��Ϊ���ݢߢ�
(3)NaHSO4��ˮ��Һ����ȫ���룬����Na+��H+��SO42-�����뷽��ʽΪ: NaHSO4=Na++H++SO42-
����NaHSO4=Na++H++SO42-��
(4)�Ʊ�������������ķ��������ˮ�еμӱ��͵�FeCl3��Һ������ѡ�⣬���ӷ���ʽΪ:Fe3����3H2O![]() Fe(OH)3(����)��3H����
Fe(OH)3(����)��3H����
��Ϊ���⣻Fe3����3H2O![]() Fe(OH)3(����)��3H����
Fe(OH)3(����)��3H����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Wԭ�ӵ����������������ڲ��������3���������ж���ȷ����
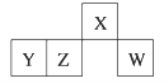
A. ԭ�Ӱ뾶��rW��rZ��rY��rX B. ��YԪ�ص�����Һһ��������
C. �����̬�⻯������ȶ��ԣ�W��X D. Z������������ˮ����������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Һ�У���������һ���ܹ������������
A��ʹ��̪��Һ������Һ��Na����Cl����SO![]() ��Fe3��
��Fe3��
B��ʹ��ɫʯ����Һ������Һ��Fe2����Mg2����MnO![]() ��Cl��
��Cl��
C��pH<7����Һ��K����Ba2����Cl����Br��
D��̼��������Һ��K����SO![]() ��Cl����H��
��Cl����H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʽ������ˮ�ⷴӦ������Һ�����Ե��� ( )
A. HCO3- + H2O ![]() H3O+ + CO32- B. Fe3+ + 3H2O
H3O+ + CO32- B. Fe3+ + 3H2O ![]() Fe(OH)3 + 3H+
Fe(OH)3 + 3H+
C. HS- + H2O ![]() H2S + OH- D. NH4+ + OH-
H2S + OH- D. NH4+ + OH- ![]() NH3��+ H2O
NH3��+ H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�з�������ͼ�����( )

A. N2(g)+3H2(g)![]() 2NH3(g) ��H=-Q1kJ��mol-1(Q1>0)
2NH3(g) ��H=-Q1kJ��mol-1(Q1>0)
B. 2SO3(g)![]() 2SO2(g)+O2(g) ��H=+Q2kJ��mol-1(Q2>0)
2SO2(g)+O2(g) ��H=+Q2kJ��mol-1(Q2>0)
C. 4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g) ��H=-Q3kJ��mol-1(Q3>0)
4NO(g)+6H2O(g) ��H=-Q3kJ��mol-1(Q3>0)
D. H2(g)+CO(g)![]() C(s)+H2O(g) ��H=+Q4kJ��mol-1(Q4>0)
C(s)+H2O(g) ��H=+Q4kJ��mol-1(Q4>0)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���״��ǿ�������Դ�����п���Ӧ�õĹ���ǰ�����ش��������⣺
һ���¶��£���һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO��g��+2H2��g��CH3OH��g��
���������β���˵���÷�Ӧ�Ѵﵽƽ��״̬����________������ţ���
A��ÿ����1mol CO��ͬʱ����2molH2 B��������������ʵ�������
C��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯 D������CH3OH������������CO���������
��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��A��B�����ƽ�ⳣ��K��A��________K��B�������������=����������

��2����֪25��ϳɰ���Ӧ�У�1mol N2��ȫת��ΪNH3ʱ�ͷŵ�����Ϊ92.4 kJ���ֽ�1mol N2��3mol H2�������2L�ܱ������У���Ӧ���е�2sĩ���NH3Ϊ0.4mol���ش��������⣺
�� �÷�Ӧ���Ȼ�ѧ����ʽ��________ ��
�� �÷�Ӧ�ﵽƽ��������¶�ƽ����________ �������Ӧ�������淴Ӧ�����ƶ����������ƽ��________������������桱�� ���������ƶ���
�� ǰ2s��v��H2����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶���,��ij�ܱ������м���0.2 mol CO��0.2 mol H2O,������ӦCO��g��+H2O��g��![]() CO2��g��+H2��g��,4 minʱ��Ӧ�ﵽƽ��״̬,���n��CO����n��CO2��=3��2,����˵����ȷ����
CO2��g��+H2��g��,4 minʱ��Ӧ�ﵽƽ��״̬,���n��CO����n��CO2��=3��2,����˵����ȷ����
A.��С���������,���������ܶȲ���
B.v��CO��=v��CO2��ʱ,������Ӧ�ﵽƽ��״̬
C.ƽ��ʱCO��H2O��ת�������
D.������������·�Ӧ�Ļ�ѧƽ�ⳣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о����̽����㺬�е�Ag���������������Բ��ƣ�����������е�Ag2O�ȷϾ���Դ�Ļ������������ش���ͼΪ�ӹ�������ȡAg�Ĺ�ҵ���̡���ش��������⡣

��1��NaClO��Һ�����Ȼ������������ֽ⣬��������������80�������½��У����˵ļ��ȷ�ʽΪ________��
��2��NaClO��Һ��Ag��Ӧ�IJ���ΪAgCl��NaOH��O2���÷�Ӧ�Ļ�ѧ����ʽΪ________�����������HNO3����NaClO����Ag���ӷ�Ӧ����ĽǶȷ�������ȱ����________��
��3�������������Ϊ____���������á�������װ��ϴ���������ʵ�����________��
��4����ѧ�ϳ���10���İ�ˮ�ܽ�AgCl���壬AgCl��NH3��H2O��1�U2��Ӧ������Cl����һ��������________����Һ���������ӵĻ�ѧʽ����ʵ�ʷ�Ӧ�У���ʹ��ˮ����Ҳ���ܽ�AgCl����ȫ���ܽ⣬���ܵ�ԭ����________��
��5������ʱN2H4��H2O��ˮ���£��ڼ����������ܻ�ԭ��4�������ɵ������ӣ�����ת��Ϊ������N2������������0.1 mol��ˮ���¿���ȡ��________g�ĵ���Ag��
��6���Ͼɵ����Ag2O�ܽ��ж������ȩ��HCHO��������CO2����ѧ�Ҿݴ�ԭ���������������Ϊԭ��ػ��յ缫����Ag����Чȥ��������ȩ����˵�ص�������ӦʽΪ________�������IJ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)���������ڱ�������Ԫ�ذ��� ______________________(��Ԫ������)����Ԫ�أ�����Se�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ______________________��Ԫ��X��Seͬ���ڣ�XԪ��ԭ�Ӻ���δ�ɶԵ�������࣬XΪ______________________(��Ԫ�ط���)��
(2)����ͬ���ڵ�����Ԫ���У���һ�������д�С��˳��______________________��
(3)�����ŷŵij�����Ҫ�ɷ�Ϊ3��MBT(3������2����ϩ���ṹ��ͼ)��1mol3��MBT�к���������ĿΪ____________(NAΪ����ӵ�������ֵ)���е㣺3��MBT____________(CH3)2C==CHCH2OH(������������������=��)����Ҫԭ����________________________��

(4)S��+4��+6���ּ�̬�������
�����й�����̬SO3��SO2��˵����ȷ����__________(�����)��
A.����ԭ�ӵļ۲���Ӷ��������
B.���Ǽ��Է���
C.����ԭ�ӵĺ˶Ե�����Ŀ�����
D.�����м��Լ�
��SO3���ӵĿռ乹��Ϊ__________�����以Ϊ�ȵ������������Ϊ__________(��һ��)��
(5)����Po����__________���γɵľ��壻����֪Po��Ħ������ΪMg��mol-1��ԭ�Ӱ뾶Ϊrpm�������ӵ�������ֵΪNA�����Ǿ�����ܶȵı���ʽΪ____________________g��cm-3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com