

| 23700m2 |
| 233m |
| 23700m2 |
| 233m |
| m2g |
| 233g/mol |
| m2 |
| 233 |
| m2 |
| 233 |
| 1 |
| 2 |
| 237m2 |
| 233 |
| ||
| mg |
| 23700m2 |
| 233m |
| 23700m2 |
| 233m |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 23700m2 |
| 233m |
| 23700m2 |
| 233m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
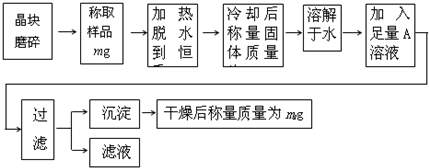
Ϊ�˲ⶨ��������KCl���ʵ�����KAl��SO4��2��nH2O�Ĵ��ȣ�ͬʱ�ⶨn��ֵ�����������̽���ʵ�飺

��1��������Ʒ����������ƽ�������й�ʹ������
��ƽ������������ȷ���� ( ).
A������ǰ�ȵ���������ƽ�����
B������ʱ���̷ű�������,���̷�����
C����ʪ�Ļ���и�ʴ�Ե�ҩƷ,������ڲ������������,��������ҩƷ��ֱ�ӷ�����ƽ�����ϳ���
D����������ƽ����ȷ������0.01��
E���������,Ӧ������Ż��������
��2���ж�����ˮ�����صķ����ǣ� ��
A����ʱ����� B�����γ�������������һ��
C���۲���ˮ��������ð�� D�����γ��������������0.1g
��3���ڼ��Ƚ�������ȴ����IJ���Ϊ������������ �� ���������� ��
��4��A��Һ���������������ѧʽ�����ж�A��Һ�Ƿ������ķ���������������������
��������������������������������������������������������������������
��5��ͨ��ʵ�飬���������ѧʽ��nֵΪ12���������Ĵ���Ϊ���� ��%��
����֪KAl��SO4��2��12H2O��ʽ��Ϊ474��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ�߿���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com