
Ϊ�˲ⶨ�������ƹ�������m g��̼���Ƶ������������ס�����λͬѧ�ֱ���������µ�ʵ�鷽����
(��)��ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ�����ˡ�ϴ�ӡ���ɣ������ù���n g��
(1)�������̼���Ƶ���������Ϊ(��m��n��ʾ)________��
(2)��ͬѧϴ�ӳ����IJ�����________________��
(3)Ca2+��Ba2+������ʹCO32��������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ���ǣ�
��________________________��
��________________________��
(��)��ͬѧ�ķ�����ͼ��ʾ��
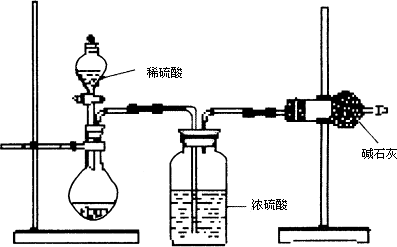
(1)������ͬѧ��ʵ��װ��ͼ��������ÿ��ʵ���У���ɲⶨ��������Ҫ����________�γ���������
(2)���ظ���ȷ���������Σ�������ݳ����˽ϴ��ƫ�����Ϊ��Ҫԭ�������(��д����)����________________________________��
��_______________________________________��
��_______________________________________��
����(��)(1)10600 n/197 m��
����(2)�ز�������������еij����ϼ�����ˮ����û����������ʹ��ȫ���˳����ظ�2��3��
����(3)�ټ���BaCl2��Һ�����ɵij�������������������С��
�����ڹ�����Ca2+����OH�������ܵ�Ca(OH)2����Ӱ��ⶨ���
����(��)(1)4
����(2)��װ����ԭ�п����е�CO2û���ų�������Ҳ����ʯ������
�����ڷ�Ӧ��ɺ�װ���е�CO2û��ȫ������ʯ������
�����ۿ����е�CO2��ˮ��������ʯ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
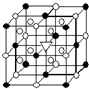 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2O2 | 272 | 423 | ������Ȼ��� |
| 80m-135n |
| 18n |
| 80m-135n |
| 18n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������һ����顡���л�ѧ(ѡ��)��ѧ��Ӧԭ�������հ�α걾 ���հ�α걾 ���ͣ�058
Ϊ�˲ⶨ�������ƺ�̼���ƹ�������m g��̼���Ƶ������������ס��ҡ�����λͬѧ�ֱ���������µ�ʵ�鷽����
(1)��ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ������ϴ�ӣ�ȡ������ɣ������ù���n g����������̼���Ƶ���������Ϊ________����Ca2+��Ba2+����ʹ![]() ������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����________��
������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����________��
(2)��ͬѧ�ķ����ǣ�����Ʒ�ܽ�����Թ������Ȼ�����Һ���ٵ���2��3��________��Һ��ָʾ�����ñ�����ζ�����ͬѧ�ڵζ�����������Ҫ����Ҫ����������________��________����������Ȼ�����Һ��Ŀ����________��
(3)��ͬѧ�ķ�����ͼ��ʾ��
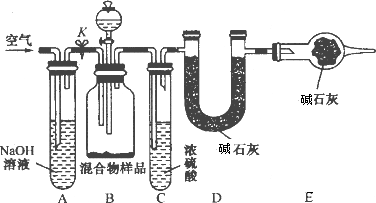
���������Ʒ��ַ�Ӧ��ȫʱ������ͨ�������Ŀ����________�����У�װ��A��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬��������Ȼ�����Һ�����ˣ�������ϴ�ӡ���ɣ��������ù�������Ϊn g��
�ټ�������Ѿ�ϴ���ķ���Ϊ____________________________________________��
�ڴ˻������̼�������������ļ���ʽΪ_________________________________________��
��2����ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬��������Ȼ�����Һ���ٵ���2��3�η�̪��Һ����a mol/L������ζ�������ζ��յ�ʱ��������b mL��
����ͬѧ�ڵζ�����������Ҫ����Ҫ����������______________��______________��
�ڴ˻������̼�������������ļ���ʽΪ______________��
��3����ͬѧ�ķ�������ͼ��ʾ��
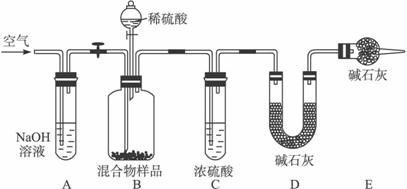
��װ��C��������____________________________________________________��
�ڵ��������Ʒ��ַ�Ӧ����ͨ�������Ŀ���ǣ�_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�˲ⶨ�������ƹ�������m g��̼���Ƶ�����������ij̽���С��ֱ����������ʵ�鷽����
��1����ͬѧ����ͼһ��ʾװ�ã�ͨ���ⶨ�ų�CO2�������ȷ��̼���Ƶ�����������������ϴ���������Ľ���ʩ ��
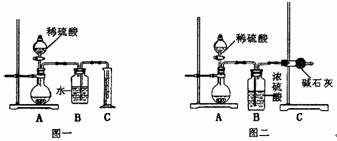
��2����ͬѧ��ͼ����ʾװ�ã�ͨ���ⶨ��Ӧ�ų�CO2��������ȷ��̼���Ƶ��������������ظ���ȷ�������Σ�������ݳ����˽ϴ�ƫ�����Ϊ��Ҫԭ������ǣ���д������
��
��
��3����ͬѧ��Ϊ����IJⶨ�������ϴ�������к͵ζ����ⶨ�������ǣ�ȷ��ȡ����������Ʒm g��������ƿ�м�����ˮ�ܽ⣬��1�D2�η�ָ̪ʾ���������ʵ���Ũ��Ϊc mol?L�D1��HCl��Һ�ζ�����Һ�ɺ�ɫ����ɫ��ָʾCO32�D+H+=HCO3�D��Ӧ���յ㣩������HCl��Һ���ΪV1mL���ټ���1�D2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻƱ�ȣ�ָʾH++HCO3�D=CO2��+H2O��Ӧ���յ㣩��������HCl��Һ���ΪV2ml�����ռ���Ʒ��Na2CO3����������Ϊ ��
��4�������������һ����ʵ�鷽�����ⶨNaOH����������Na2CO3������������
�����������
���ò�õ����ݣ�����ĸ��ʾ��д�����������ı���ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com