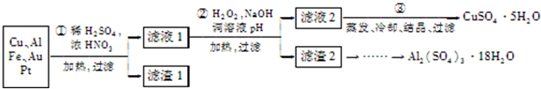
������Cu��Al������Fe��Au��Pt�Ƚ����Ļ�����м��������Ũ����Ļ�����ܽ⣬���Եõ����Ρ����Ρ�ͭ�εȣ�Au��Pt�Ȳ��������˳�������Һ�м���˫��ˮ���������ƣ���H
2O
2�������ǰ�Fe
2+����ΪFe
3+�������������ŵ��Dz��������ʣ�����Ի�������Ⱦ������ҺPH��Ŀ����ʹFe
3+��Al
3+�γɳ���������ͼ�����ݷ�����֪����ҺPH����Ϊ5.2��5.4��ʹFe
3+��Al
3+�γɳ�����ͭ���Ӳ�����������������м����������ƿ���ʵ�������������������Ƶķ��룬���������м�NaOH��Al��OH��
3��Ӧ����NaAlO
2��������Һ�м�H
2SO
4����Al
2��SO
4��
3����������ȴ���ᾧ�����˿ɵ����������壮
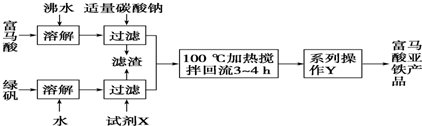
��1����������������������ԭΪˮ����������Ⱦ���������������ᱻ��ԭ���ɵ���������Ⱦ������ͭ������Һ�б�������������Ϊͭ���ӣ���������ԭΪˮ������ԭ���غ㡢����غ���ƽд�����ӷ���ʽ��
��2���������̷�������������������������Ϊ�����Ӻ����������Ƶ�����ҺPHʹ�����Ӻ�������ȫ��������ͭ���Ӳ�������
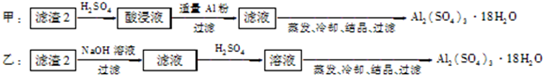
��3������2�ijɷ�Ϊ������������������������ʵ�鷽�����̷����Ʊ��������Ƿ������ʣ�ʹ�õ��Լ����ã�ԭ�ϵ������ʣ�ԭ�����������ط����жϣ�
��4���ٸ��ݳ����ܽ�ƽ�����д�������ش�
���ȸ���CuS���ܶȻ�K
sp=9.0��10
-36����������ӵ�Ũ�ȣ�����Һ�����Ϊ1L������ܽ��CuS��������Ȼ�����
=
���CuS�ڸ��¶��µ��ܽ�ȣ�
��Ksp=c��Cu
2+��?c��S
2-����ͭ���ӵ�Ũ�ȴﵽ1��10
-5mol/L����Ϊ������ȫ���ݴ˼��㣮
���
�⣺Cu��Al������Fe��Au��Pt�Ƚ����Ļ�����м��������Ũ����Ļ�����ܽ⣬���Եõ����Ρ����Ρ�ͭ�εȣ�Au��Pt�Ȳ��������˳�������Һ�м���˫��ˮ���������ƣ���H
2O
2�������ǰ�Fe
2+����ΪFe
3+�������������ŵ��Dz��������ʣ�����Ի�������Ⱦ������ҺPH��Ŀ����ʹFe
3+��Al
3+�γɳ���������ͼ�����ݷ�����֪����ҺPH����Ϊ5.2��5.4��ʹFe
3+��Al
3+�γɳ�����ͭ���Ӳ�����������������м����������ƿ���ʵ�������������������Ƶķ��룬���������м�NaOH��Al��OH��
3��Ӧ����NaAlO
2��������Һ�м�H
2SO
4����Al
2��SO
4��
3����������ȴ���ᾧ�����˿ɵ����������壮
��1����������������������ԭΪˮ����������Ⱦ��ͭ������Һ�б�������������Ϊͭ���ӣ���������ԭΪˮ����Ӧ�����ӷ���ʽΪ��Cu+H
2O
2+2H
+�TCu
2++2H
2O��
�ʴ�Ϊ��Cu+H
2O
2+2H
+�TCu
2++2H
2O��
��3����H
2O
2�������ǰ�Fe
2+����ΪFe
3+�������������ŵ��Dz��������ʣ�����Ի�������Ⱦ������ҺPH��Ŀ����ʹFe
3+��Al
3+�γɳ���������ͼ�����ݷ�����֪����ҺPH����Ϊ5.2��5.4��ʹFe
3+��Al
3+�γɳ�����ͭ���Ӳ�������������Һ2�ijɷ���Cu
2+������2�ijɷ�Ϊ��������������������
�ʴ�Ϊ��5.2��5.4��
��4���Ʊ�����������ļס������ַ����У��������������м�H
2SO
4������Fe
2��SO
4��
3��Al
2��SO
4��
3���ټ�Al�ۺ�Fe
2��SO
4��
3����Al
2��SO
4��
3����������ȴ���ᾧ�����˿ɵ����������壻�ҷ������������м�NaOH��Al��OH��
3��Ӧ����NaAlO
2��������Һ�м�H
2SO
4����Al
2��SO
4��
3����������ȴ���ᾧ�����˿ɵ����������壻����ԭ�����ýǶȿ��Ƿ��������������Ϊ�Ҽӵ�NaOH���Ʊ���Al
2��SO
4��
3��ԭ�����û�й�ϵ�����ԭ���˷ѣ�
�����������ַ����У���ԭ�������ʺ��Ƿ�������ʿ���֪��������������
�ʴ�Ϊ���ף��õ���Ʒ���ȸߡ�ԭ�������ʸߣ�
��4����CuS�����ܽ�ƽ��ı���ʽΪ��CuS��S��?Cu
2+��aq��+S
2-��aq����
�ʴ�Ϊ��CuS��S��?Cu
2+��aq��+S
2-��aq����
��CuS���ܶȻ�K
sp=9.0��10
-36����C��Cu
2+��=3��10
-18mol/L������Һ�����Ϊ1L��n��CuS��=n��Cu
2+��=3��10
-18mol/L��1L=3��10
-18mol���ܽ��CuS������Ϊ3��10
-18mol��96g/mol=2.88��10
-16g����Һ������Ϊ��1000ml��1g?mL
-1=1000g��ˮ������Ϊ1000g-2.88��10
-16g��1000g��CuS�ڸ��¶��µ��ܽ����
=2.88��10
-17g��
�ʴ�Ϊ��2.88��10
-17g��
��CuSO
4��Һ�м���Na
2S��������Ҫʹ��Һ��Cu
2+������ȫ����ͭ���ӵ�Ũ�ȴﵽ1��10
-5mol/L����Ϊ������ȫ������Һ�е�C��S
2-��=
=
mol/L=9��10
-31mol/L��
�ʴ�Ϊ��9��10
-31mol/L��



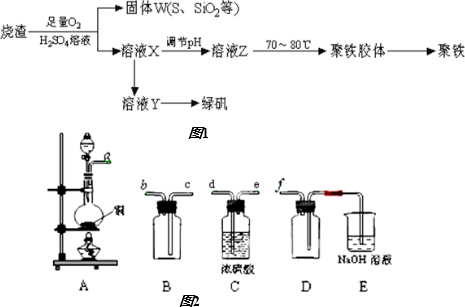
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
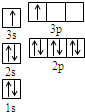 A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1�� Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ�EΪ��������δ�ɶԵ���������Ԫ�أ���ش��������⣺
A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1�� Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ�EΪ��������δ�ɶԵ���������Ԫ�أ���ش��������⣺ ����һ������ʹ�õ���ǿ������
����һ������ʹ�õ���ǿ������