
���� ��1��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų��������������Ȼ�ѧ����ʽ����д����д������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ䣻
��2�����ݲ�ͬ�ۼ�״̬��ˮ��������ͬ���Լ������ߵͽ����жϣ�
��3��1mol������ȫȼ��������̬ˮ�ų�242kJ������1molҺ̬ˮ����Ϊ��̬ˮ��Ҫ����44kJ��������д�Ȼ�ѧ����ʽ��ϸ�˹���ɼ���õ���
��4��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų���������1mol����ȼ������Һ̬ˮ�ų�������Ϊȼ���ȣ�
��5��1molCO������ȫȼ������CO2����ų�283kJ��������дCOȼ���ȵ��Ȼ�ѧ����ʽ��1molCH4������ȫȼ������CO2�����Һ̬ˮ�ų�890kJ������д�Ȼ�ѧ����ʽ����ϸ�˹���ɼ���õ���
��� �⣺��1��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų���������1molCO������ȫȼ������CO2����ų�283kJ��������ʾCOȼ���ȵ��Ȼ�ѧ����ʽΪ��CO��g ��+$\frac{1}{2}$ O2��g ��=CO2��g����H=-283 kJ/mol��
�ʴ�Ϊ��CO��g ��+$\frac{1}{2}$ O2��g ��=CO2��g����H=-283 kJ/mol��
��2��ˮ����̬��ΪҺ̬ʱҪ���ȣ�1mol CH4������ȫȼ������CO2�����Һ̬ˮ�ų�890KJ��������1mol CH4������ȫȼ������CO2�����ˮ�������ų�������С��890kJ���ʴ�Ϊ������
��3��H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-242kJ/mol��H2O��l��=H2O��g����H=+44kJ/mol��ȼ��1mol��������Һ̬ˮ���Ȼ�ѧ����ʽΪ��H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286kJ/mol��ȼ��10gH2����Һ̬ˮ����$\frac{10g}{2g/mol}$��286KJ/mol=1430KJ��
�ʴ�Ϊ��1430 kJ��
��4��H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-242kJ/mol��H2O��l��=H2O��g����H=+44kJ/mol��1mol������ȫȼ������Һ̬ˮ�ų�286kJ����������ȼ���ȵ��Ȼ�ѧ����ʽΪ��H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286kJ/mol��
�ʴ�Ϊ��286 kJ/mol��
��5��1molCO������ȫȼ������CO2����ų�283kJ��������дCOȼ���ȵ��Ȼ�ѧ����ʽΪ����CO��g ��+$\frac{1}{2}$ O2��g ��=CO2��g������H=-283 kJ/mol��1molCH4������ȫȼ������CO2�����Һ̬ˮ�ų�890kJ��������CH4��g ��+2O2��g ��=CO2��g��+2H2O��l����H=-890 kJ/mol�����ݸ�˹���ɢ�+�ڵõ���CH4��g ��+$\frac{3}{2}$O2��g ��=CO��g��+2H2O��l����H=-607kJ/mol��
�ʴ�Ϊ��CH4��g ��+$\frac{3}{2}$O2��g ��=CO��g��+2H2O��l����H=-607kJ/mol��
���� ���⿼�����Ȼ�ѧ����ʽ��д��������˹���ɵļ���Ӧ�ã���Ҫ�����ʾۼ�״̬��ȼ���ȸ��������Ӧ�ã���Ŀ�Ѷ��еȣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۻ� | B�� | ��ɫ��Ӧ | C�� | �绯 | D�� | �������Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
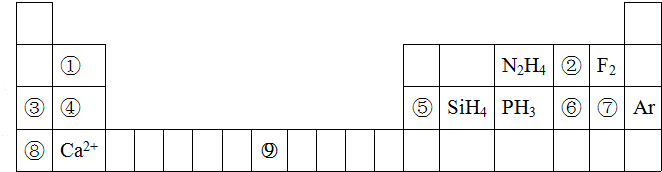
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ϩ�� | C�� | ������ | D�� | ±���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ���� | C�� | ��֬ | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������SO42-Ũ�������� | |
| B�� | ����ͨ��������ͭƬ����пƬ | |
| C�� | ������ӦʽΪZn-2e-�TZn2+ | |
| D�� | ��ԭ��ع��������е������Һ��pHֵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com