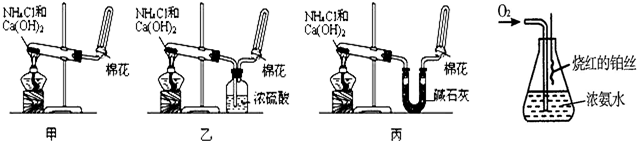
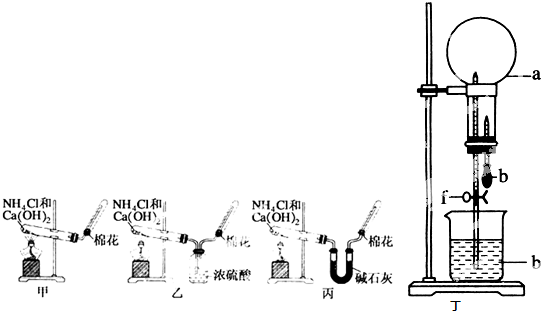
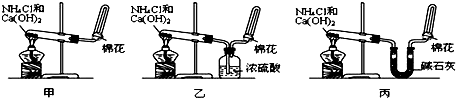
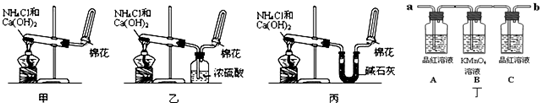
�ס��ҡ�����λͬѧ�ֱ�������ʵ��װ�ü���ѧҩƷ����ʯ��Ϊ�������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش����⣺
��1����ȡ�����Ļ�ѧ����ʽΪ��______��
��2����λͬѧ���������ſ������ռ���������ԭ����______��
��3����λͬѧ������װ����ȡ����ʱ��������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��______����ס������ҡ���������
��4�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ���______��
��5����λͬѧ����Ϊ�������������Ե�װ�ã��������ڼ���̼����粒���ķ�������ȡ�����İ���������Ϊ��λͬѧ�ܹ��ﵽʵ��Ŀ��______����ס������ҡ������������ǻ���Ϊ��װ���е�NH
4HCO
3�������NH
4Cl������棬����Ϊ______����ܡ����ܡ�����
��6��ij����С������й�����֪����˿�ǰ������Ĵ���������ƽ�������ͼʵ�飮ʵ������й۲쵽��ƿ�������Ϊ����ɫ��ƿ�ڳ��ְ���
д����ʵ������л�ѧ��Ӧ����ʽ��______��______��______��______��