
 a2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����H2O2������ˮ��
a2CO3��NaHCO3��MnO2��Na2O2��NaCl����ˮCaCl2��NH4HCO3����ʯ�ҵȹ����H2O2������ˮ��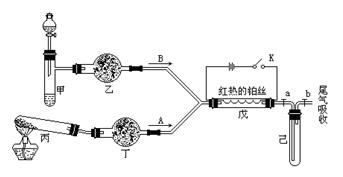
 ��
�� �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��װ�â�����֤AgCl������ת��Ϊ�ܽ�ȸ�С��Ag2S���� |
| B��װ�â���X��Ϊ���Ȼ�̼�����������հ���������ֹ���� |
| C��װ�â۵�ʵ����ƶ���̼��������Ԫ�صķǽ�����ǿ�� |
| D��װ�âܿɼ��������鷢����ȥ��Ӧ�õ��������к�����ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
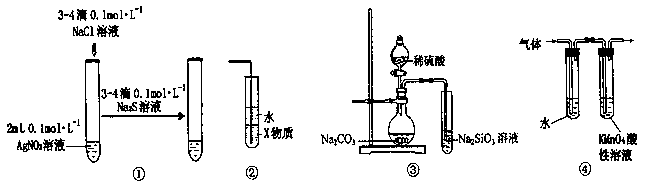
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
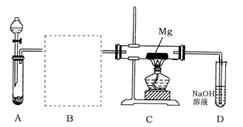
 Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺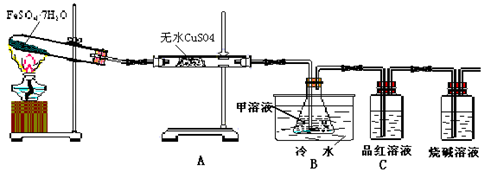
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ� ���� | ������Һ����� /mL | ����Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
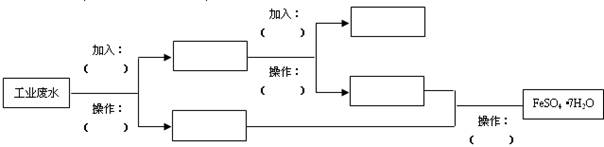
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | NaOH��ʼ���� | NaOH�յ���� |
| ��һ�� | 0.40mL | 18.50mL |
| �ڶ��� | 1.30mL | 18.05mL |
| ������ | 3.10mL | 21.20mL |
 ɫ�� ɫ��
ɫ�� ɫ��| A���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ |
| B��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ |
| C���ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ |
| D��δ�ñ�Һ��ϴ��ʽ�ζ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �а��Ĵ��������ش��������⣺
�а��Ĵ��������ش��������⣺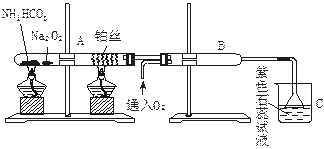
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com