
��1�����к��ȵIJⶨʵ���У�ȡ50 mL 0.50 mol /L�����ᣬ��������� �Լ�������ţ�����50 mL0.50 mol/L NaOH��Һ ��50 mL0.55 mol/L NaOH��Һ ��1.0 g NaOH����
 ��2��������ͭ����ᾧˮ�����IJⶨʵ���У�������������Ϊm������������ͭ���������Ϊm1�����Ⱥ������������ˮ����ͭ������Ϊm2�������нᾧˮ�����������أ�____________________��д����ʽ�������ʵ���м����¶ȹ��ߣ�������ɫ��ڣ���ⶨ�����____________������䡱����ƫ�ߡ�����ƫ�͡�����
��2��������ͭ����ᾧˮ�����IJⶨʵ���У�������������Ϊm������������ͭ���������Ϊm1�����Ⱥ������������ˮ����ͭ������Ϊm2�������нᾧˮ�����������أ�____________________��д����ʽ�������ʵ���м����¶ȹ��ߣ�������ɫ��ڣ���ⶨ�����____________������䡱����ƫ�ߡ�����ƫ�͡�����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������������2005��������ԡ�������ѧ�Ծ� ���ͣ�022
���к��ȵIJⶨʵ���У�ȡ50 mL 0.50 mol /L�����ᣬ���������________�Լ�(�����)����50 mL0.50 mol/L NaOH��Һ������50 mL0.55 mol/L NaOH��Һ������1.0 g NaOH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���ֶ���2006-2007ѧ����ѧ�����п��ԡ�������ѧ���� ���ͣ�022
(1)ͼ���ʾ10mL��Ͳ��Һ���λ�ã�A��B�̶ȼ����1mL������̶�AΪ4����Ͳ��Һ��������________mL��
(2)ͼ���ʾ50mL�ζ�����Һ���λ�ã����Һ�洦�Ķ�����a����ζ�����Һ������(�����)________��
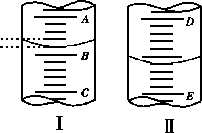
A����amL
B����(50��a)mL
C��һ������amL
D��һ������(50��a)mL
(3)��ѡ��CCl4�ӵ�ˮ����ȡ���ԭ����________��
(4)���к��ȵIJⶨʵ���У�ȡ50mL0.50mol/L�����ᣬ���������________�Լ�(�����)��
��50mL0.50mol/LNaOH��Һ��50mL0.55mol/LNaOH��Һ��1.0gNaOH����
(5)������ͭ����ᾧˮ�����IJⶨʵ���У�������������Ϊm������������ͭ���������Ϊm1�����Ⱥ������������ˮ����ͭ������Ϊm2�������нᾧˮ�����������أ�________(д����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��Ҵ��ѧ2007������꼶ģ��ѵ������ѧ�Ծ� ���ͣ�043
| |||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ���¶��и߿�һ�ָ�ϰ�ο��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��1�����к��ȵIJⶨʵ���У�ȡ50 mL 0.50 mol /L�����ᣬ��������� �Լ�������ţ�����50 mL0.50 mol/L NaOH��Һ ��50 mL0.55 mol/L NaOH��Һ��1.0 g NaOH����
��2��������ͭ����ᾧˮ�����IJⶨʵ���У�������������Ϊm������������ͭ���������Ϊm1�����Ⱥ������������ˮ����ͭ������Ϊm2�������нᾧˮ�����������أ�____________________��д����ʽ�������ʵ���м����¶ȹ��ߣ�������ɫ��ڣ���ⶨ�����____________������䡱����ƫ�ߡ�����ƫ�͡�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com