
����Ŀ������ϩ������Ǻϳ��л������ĵ��塣��|���ϳɵķ�Ӧ�ǣ�
��(CH3)2C=O+HCN��(CH3)2C(OH)CN
��(CH3)2C(OH)CN+CH3OH+H2SO4��CH2=C(CH3)COOCH3+NH4HSO4
�·��ϳɵķ�Ӧ��:��CH3C��CH+CO+CH3OH![]() CH2=C(CH3)COOCH3
CH2=C(CH3)COOCH3
����˵������ȷ����
A.����ϩ����������ֹ�����B.��Ӧ�۷�����ɫ��ѧ˼��
C.��Ӧ����CH3OH��CH2OHCH2OH(�Ҷ���)��ͬϵ��D.��Ӧ���Ǽӳɷ�Ӧ
���𰸡�C
��������
A�����ݼ���ϩ������Ľṹ��ʽΪCH2=C(CH3)COOCH3���ýṹ����̼̼˫�����������ֹ����ţ���A��ȷ��
B���÷�Ӧֻ��һ��Ŀ�������Է�����ɫ��ѧ˼�룬��B��ȷ��
C���״��к���1��-OH���Ҷ�������2��-OH�����߽ṹ�����ƣ����Բ�����ͬϵ���C����
D������̼̼˫��������������̼��˫�����л����ij�������£�˫�����������е�һ�����ۼ��Ͽ���Ȼ����ԭ���γ�˫��������ԭ���Ϸֱ���������������ԭ�ӻ�ԭ���ŵķ�Ӧ�������ӳɷ�Ӧ�����Է�Ӧ�ٷ��ϼӳɷ�Ӧ����D��ȷ��
��ΪC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(NaN3)������������ȫ�����е�ҩ����ʵ������ȡ�������Ƶ�ԭ����ʵ��װ�ü�ʵ�鲽�����£�
�ٴ�װ��D�����ϵ�������������ȡ������
���ټ���װ��A�еĽ����ƣ�ʹ���ۻ�����ַ�Ӧ����ֹͣ����װ��D���ر�������
����װ��A��b�����ڳ�����Ƚ��ʲ����ȵ�210��220�棬Ȼ��ͨ��N2O��
����ȴ��������м����Ҵ�(����NaN3���ܽ��)����ѹŨ���ᾧ���ٹ��ˣ���������ϴ�ӣ����ɡ�

��֪��I��NaN3��������ˮ�İ�ɫ���壬�����Ҵ������������ѣ�
II��NaNH2�۵�210�棬�е�400�棬��ˮ��Һ����ˮ�⡣
��ش��������⣺
(1)װ��B��ʢ�ŵ�ҩƷΪ_____________��װ��C����Ҫ������______________________��
(2)��������ȼ���ͨ������Ŀ����_____________________________________������ڰ������ۻ����Ʒ�Ӧ����NaNH2�Ļ�ѧ����ʽΪ_______________________________��������������˵ļ��ȷ�ʽΪ ___________(����ˮԡ������������ԡ������)��
(3)N2O����NH4NO3��240��245��ֽ��Ƶ�(����淋��۵�Ϊ169.6��)�����ѡ������巢��װ����(�����)___________��

(4)����NaN3�Ļ�ѧ����ʽΪ _____________________________________��
(5)ͼ������a�õ������ʶ����ò���������Ҫԭ����__________________��
(6)�������������ϴ�ӵ���ҪĿ����_______________________________��
(7)ʵ�����õζ����ⶨ����������Ʒ��NaN3�������������ٽ�2.500 g�������500.00 mL��Һ����ȡ50.00 mL��Һ������ƿ�У�����50.00 mL 0.1010 mol��L-1(NH4)2Ce(NO3)6��Һ���۳�ַ�Ӧ����Һ��ϡ�ͣ�����Һ�м���8 mLŨ���ᣬ����3���ڷƆ���ָʾҺ����0.0500mol��L-1(NH4)2Fe(SO4)2����Һ�ζ�������Ce4+��������Һ���Ϊ29.00mL���ⶨ���̵ķ�Ӧ����ʽΪ��2(NH4)2Ce(NO3)6+2NaN3=4NH4NO3+2Ce(NO3)3+2NaNO3+3N2����Ce4++Fe2+=Ce3++Fe3+��������NaN3����������Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����û�ѧ��Ӧԭ���о�̼��������ĵ��ʼ��仯����ķ�Ӧ�Ի������Ⱦ����ԴΣ��������Ҫ���塣
��1��CO��ԭNO�ķ�ӦΪ2CO(g)+2NO(g)![]() 2CO2(g)+N2(g)H=-746kJ��mol-1��
2CO2(g)+N2(g)H=-746kJ��mol-1��
д���������������NOƽ��ת���ʵĴ�ʩ______________��______________��
��2���ý�̿��ԭNO�ķ�ӦΪ��2NO(g)+C(s)![]() N2(g)+CO2(g)
N2(g)+CO2(g)
H�����ݺ��������£��������ͬ�ļס��ҡ������������зֱ���������Ľ�̿��һ������NO����ø�������NO�����ʵ���[n(NO)]�淴Ӧʱ�䣨t���ı仯��������ʾ��
t/min n(NO)/mol ���� | 0 | 40 | 80 | 120 | 160 |
��/400�� | 2.00 | 1.5 | 1.10 | 0.80 | 0.80 |
��/400�� | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
��/T�� | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
��H______________0����������������������
����������160minʱ��v��_________v������������������������=������
��3��ij�¶��£������Ϊ2L�ĺ������������ͨ��2.0molNO2��������Ӧ��2NO2(g)![]() N2O4(g)H=-57.0kJ��mol-1����֪��v��(NO2)=k1��c2(NO2)��v��(N2O4)=k2��c(N2O4)������k1��k2Ϊ���ʳ��������NO2���������[x(NO2)]�뷴Ӧʱ�䣨t���Ĺ�ϵ�����
N2O4(g)H=-57.0kJ��mol-1����֪��v��(NO2)=k1��c2(NO2)��v��(N2O4)=k2��c(N2O4)������k1��k2Ϊ���ʳ��������NO2���������[x(NO2)]�뷴Ӧʱ�䣨t���Ĺ�ϵ�����
t/min | 0 | 20 | 40 | 60 | 80 |
x(NO2) | 1.0 | 0.75 | 0.52 | 0.50 | 0.50 |
��![]() ����ֵΪ______________��
����ֵΪ______________��
����֪���ʳ���k���¶����߶������������¶Ⱥ�k1����ı���___________k2����ı�������������������������=������
��4���ü�ӵ绯ѧ����ȥNO�Ĺ��̣���ͼ��ʾ��
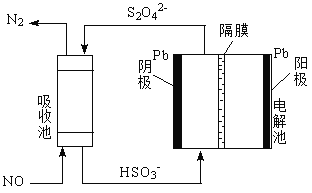
����֪���ص�����������Һ��pH��4~7֮�䣬д�������ĵ缫��Ӧʽ��______________��
�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йؾ���Ľṹ��ͼ��ʾ������˵���в���ȷ���ǣ� ��

A. ��NaCl��������Na+�����Cl-��6��
B. ��CaF2�����У�ÿ������ƽ��ռ��4��Ca2+
C. �ڽ��ʯ�����У�̼ԭ����̼̼�������ı�Ϊ1��2
D. ����̬�Ŵط��ӵķ���ʽΪEF��FE
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ����ػ������ڻ��������ϵ��������Ź㷺��Ӧ�á��ش��������⣺
��1����̬Cuԭ���У��������ռ�ݵ�����ܲ�ķ�����___________��ռ�ݸ��ܲ���ӵĵ���������ͼ��״Ϊ__________��Cu2+�۲���ӵĵ����Ų�ͼΪ___________��
��2�����Ӿ�����Cu2O�۵��Cu2S�۵�ߣ���ԭ����___________________________��
��3��Cu����N��S��O��Ԫ���γɻ����N��S��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ______________����Cu�Ĵ������£��Ҵ��ɱ�����Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��________________��
��4��1mo[Cu(NH3)4]SO4�к�����������ĿΪ___________��
��5��Cu��N���γɵ�ij�־��������������ͼ��ʾ���侧������Ϊanm���þ���Ļ�ѧʽΪ___________��������ܶ�Ϊ___________g��cm��3(�г�����ʽ���ɣ���NA��ʾ�����ӵ�����)��
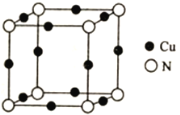
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£�20mL0.40mol/LH2O2��Һ�������ֽ⣮��ͬʱ�̲������O2�������������Ϊ��״���������
t/min | 0 | 2 | 4 | 6 | 8 | 10 | ||||
V��O2��/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ���ǣ���Һ����仯���Բ��ƣ�
A.0��6min��ƽ����Ӧ���ʣ�v(H2O2)��1.67��10-2mol/��Lmin��
B.6��10min��ƽ����Ӧ���ʣ�v(H2O2)��1.67��10-2mol/��Lmin��
C.��Ӧ��6minʱ��c(H2O2)=0.30mol/L
D.��Ӧ��6minʱ��H2O2�ֽ���50%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ҩ��������ᣨF���ĺϳ�·������ͼ��ʾ����ش��������⣺
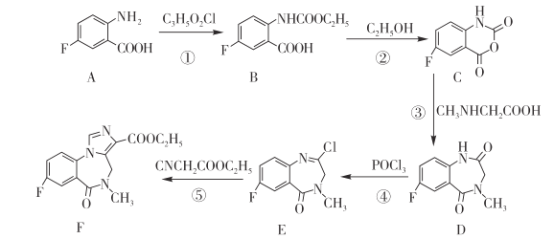
��1��A�й������з�ԭ�ӡ�_____��________�����������ƣ�
��2��C3H5O2Cl�ĽṹʽΪ________��
��3����Ӧ�ٺ͢ڵķ�Ӧ������ͬ���䷴Ӧ������___________��
��4�������� D�ķ���ʽΪ___________��
��5����Ӧ�������� ���� F�� �� HCl���� E��F�Ļ�ѧ��Ӧ����ʽΪ________��
��6��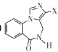 �� F��ͬ���칹�壬���� X���ֺ���COOH��û��֧���������������ͬ���칹����______�֣������������칹����
�� F��ͬ���칹�壬���� X���ֺ���COOH��û��֧���������������ͬ���칹����______�֣������������칹����
��7����֪������֮����ˮ�ܹ��γɺ��ļ��Ļ����������ɸʰ��ᣨHOOCCH2NH2����CNCH2COOC2H5�Ʊ�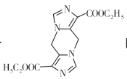 �ĺϳ�·��________�����Լ���ѡ����
�ĺϳ�·��________�����Լ���ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ҹ����гɹ����漰�����У���Ҫ�ɷ�Ϊͬ����Ԫ���γɵ����ǽ������ϵ��ǣ� ��
|
|
|
|
A.4.03�״�ھ�̼���跴�侵 | B.2022�궬�»�۰����ٻ��� | C.�����ε�Ų���̼������������ | D.�����ö������ѺϽ�ɸ���� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ֱ�߽��㴦��ԲȦΪNaCl������Na����Cl��������λ�ã������������ڿռ��������ഹֱ�ķ����϶��ǵȾ������еġ�

(1)�뽫���д���Na����ԲȦͿ��(���ؿ��������С)�������NaCl����ṹʾ��ͼ��________
(2)�����У���ÿ��Na������Χ������ӽ����Ҿ�����ȵ�Na������________����
(3)������ÿһ���ظ��Ľṹ��Ԫ�о�������NaCl��������������Ķ����ϡ����ϡ����ϵ�Na����Cl��Ϊ�þ����������ڵľ��������У�һ��������Cl���ĸ�������__����(�����ʽ)____��Na���ĸ�������____����(�����ʽ)____��
(4)��NaCl��Ħ������ΪMr g��mol��1��ʳ�ξ�����ܶ�Ϊ�� g��cm��3�������ӵ�������ֵΪNA��ʳ�ξ���������������������������ļ�ľ���Ϊ______cm��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com