
��6�֣����á���ѧ������ʵ���е�Ӧ�á������֪ʶ�������
��1������6.02��1023����ԭ�ӵ�H2SO4�����ʵ�����_________?
��2����4 g NaOH�ܽ���10 mLˮ�У���ϡ�ͳ�1 L������ȡ��10 mL����10 mL��Һ�����ʵ���Ũ��Ϊ_______��
��3����50 mL 0.1 mol��L��1 NaCl��50 mL 0.5 mol��L��1 CaCl2��Һ��Ϻ�����Һ�����Ϊ�������֮�ͣ�������Һ��c (Cl��)Ϊ?______________��
��ÿ��2�֣���6�֣���1��0.25mol (2)0.1mol/L (3)0.55mol/L
���������������1������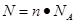 ��֪������6.02��1023����ԭ�����ʵ�����
��֪������6.02��1023����ԭ�����ʵ�����
6.02��1023��6.02��1023/mol��1mol�����Ը�������Ļ�ѧʽH2SO4��֪����������ʵ�����1mol��4��0.25mol��
��2������n��m/M��֪��4g�������Ƶ����ʵ�����4g��40g/mol��0.1mol������c��n/V��֪������Һ��Ũ����0.1mol��1L��0.1mol/L��������Һ�Ǿ�һ�ģ�����ȡ����10ml��Һ��Ũ�Ȼ���0.1mol/L��
��3�����ǰ�Ȼ��ƺ��Ȼ����������ӵ����ʵ����ֱ���0.05L��0.1mol/L��0.005mol��0.05L��0.5mol/L��2��0.05mol�����Ի�Ϻ������ӵ����ʵ�����0.005mol��0.05mol��0.055mol������Һ�������100ml�����Ի�Ϻ�������Ũ����0.055mol��0.1L��0.55mol/L��
���㣺�������ʵ������йؼ���
�������ڽ������ʵ������йؼ���ʱ���ؼ�������Ӧ�ü�����ϵʽ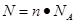 ��n��m/M��
��n��m/M�� ��
��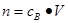 ���ر�Ҫע������Ħ������Ͱ����ӵ����ɵ�ʹ����������ֻ�����������壬��ֻ���ڱ�״���£������Ħ���������22.4L/mol������Ǽ�����Һ������Ũ�ȣ�����Ҫע�����ʵĻ�ѧʽ�Լ���Ӧ�ĵ��뷽��ʽ������ȷ����ij����Ũ�ȡ�����Ǽ������ӵ����ʵ���������Ҫע����Һ������仯��
���ر�Ҫע������Ħ������Ͱ����ӵ����ɵ�ʹ����������ֻ�����������壬��ֻ���ڱ�״���£������Ħ���������22.4L/mol������Ǽ�����Һ������Ũ�ȣ�����Ҫע�����ʵĻ�ѧʽ�Լ���Ӧ�ĵ��뷽��ʽ������ȷ����ij����Ũ�ȡ�����Ǽ������ӵ����ʵ���������Ҫע����Һ������仯��


 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| m |
| M |
| m |
| M |
| 2mNA |
| M |
| 2mNA |
| M |
| 22.4m |
| M |
| 22.4m |
| M |
| m |
| (m+1000) |
| m |
| (m+1000) |
| 18V |
| 11.2 |
| 18V |
| 11.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| m |
| M |
| m |
| M |
| 2MNA |
| M |
| 2MNA |
| M |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ��������ɽ���и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣�������mgij���壬����˫ԭ�ӷ��ӹ��ɣ�����Ħ������ΪMg��mol��1���������ӵ�������NA��ʾ����
(1)����������ʵ���Ϊ______ __
(2)����������ԭ������Ϊ ����
���á���ѧ������ʵ���е�Ӧ�á������֪ʶ�������
(3)����6.02��1023����ԭ�ӵ�H2SO4�����ʵ�����_______
(4)���״����VLCO2������ԭ����Ŀ��ͬ��ˮ��������_____ __
(5)��һ�����¶Ⱥ�ѹǿ�£�1���X2��g����3���Y2��g����������2����������û�����Ļ�ѧʽ��__________ _____
(6)�������εĻ����Һ�к���0.2 mol Na+��0 .25 mol M
.25 mol M g2+��0.4 mol Cl����SO42������n��SO42����Ϊ___ ________
g2+��0.4 mol Cl����SO42������n��SO42����Ϊ___ ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�����ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�������
��6�֣����á���ѧ������ʵ���е�Ӧ�á������֪ʶ�������
��1������6.02��1023����ԭ�ӵ�H2SO4�����ʵ�����_________?
��2����4 g NaOH�ܽ���10 mLˮ�У���ϡ�ͳ�1 L������ȡ��10 mL����10 mL��Һ�����ʵ���Ũ��Ϊ_______��
��3����50 mL 0.1 mol��L��1 NaCl��50 mL 0.5 mol��L��1 CaCl2��Һ��Ϻ�����Һ�����Ϊ�������֮�ͣ�������Һ��c (Cl��)Ϊ?______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com