
����Ŀ��ʵ������Ҫ480mL1 mol��L��1 NaOH��Һ��������Һ��������ش��������⣺
��1��ʵ���г���������ƽ(����)��ҩ�ס���Ͳ���ձ������������Ҫ������������____��
��2����ͼ��ijͬѧ��ʵ�������Ƹ�NaOH��Һ�Ĺ���ʾ��ͼ�������д������____���������ţ���
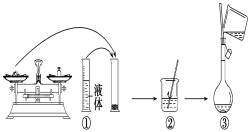

��3����ȡNaOH����ʱ���������������Ϊ____����д��ĸ����
A. 19.2g B. 20g C. 19.2g ~20g D. ����20g
��4������Һ�����ƹ����У������»���ʵ�鲽�裬����ֻ�����һ�εIJ���������ǣ���д�����������ţ�___ ��
�ٳ��� ���ܽ� ��ת�� ��ϴ�� �ݶ��� ��ҡ��
��5�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ���____��
A������NaOH��Һʱ��NaOH�����к���Na2O����
B��������ˮ�ܽ�NaOH���������ת������ƿ�ж���
C�����ݺ���Һ����ڿ��ߣ����ý�ͷ�ιܽ������ˮ����
D������ʱ���ӿ̶���
E����������ƽ���� NaOH ����ʱ����������
F������ǰ����ƿ��������ˮ��
��6����ijͬѧ���ܶ�Ϊ1.2g/cm3����������Ϊ36.5%��Ũ��������100mL3mol/L��ϡ���ᣬ��Ҫ����Ͳ��ȡŨ��������Ϊ____mL��
���𰸡���ͷ�ιܡ�500mL����ƿ �٢ۢ� D �ڢ� ABE 25.0
��������
��1�����������м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬���ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2��3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܡ������ṩ��������֪������������500ml����ƿ����ͷ�ιܣ��ʴ�Ϊ����ͷ�ιܡ�500mL����ƿ��
��2������ͲΪ��ȡ���������������ܽ����ʣ��ʢٴ���
���ò�����������ٹ�����ܽ⣬�ʢ���ȷ��
�۲�������������ʱ���������¶�Ӧ��������ƿ�̶����·����ʢ۴���
�ܼ�ˮ���̶��ߵ��·���������ȷ���ʢ���ȷ��
�ݶ���ʱ���۾�Ӧƽ�ӿ̶��ߣ��ʢݴ���
�Ӹ�ҡ�ȣ�ʹ��Һ��Ͼ��ȣ�������ȷ���ʢ���ȷ��
�ʴ�Ϊ���٢ۢݣ�
��3��������480mL������ƿ����ѡ��500mL������ƿ�����Ƴ�500mL��1.0mol/L����Һ��500mL 1.0mol/L������������Һ�к������ʵ�����Ϊ��m��1.0mol/L��0.5L��40g/mol��20g����Ҫ�������������Ƶ�����Ϊ20.0g���������������ƹ���ʱ��Ҫ�ŵ�С�ձ����������ѡ�õ��������������20.0g���ʴ�Ϊ��D��
��4���ٳ���ʱ�ȳƿ��ձ��������ٳ��ձ���ҩƷ���������ʢٴ���
�ڹ������ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У�ֻ��1�Σ��ʢ���ȷ��
��ת��ʱ���˽���Һת�Ƶ�����ƿ�л�Ҫ��ϴ��Һת�Ƶ�����ƿ�У��ʢ۴���
��ϴ��ʱҪϴ���ձ���������2��3�Σ��ʢܴ���
�ݶ���ʱ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�ʹ��Һ�İ�Һ�����͵��������ƽ��ֻ��1�Σ��ʢ���ȷ��
��ҡ��Ҫ����Һ��ҡ��һ�Σ��ڶ��ݺ���ҡ��һ�Σ��ʢ���
��ѡ���ڢݣ�
��5��A.NaOH�����к���Na2O���ʣ�Na2O����ˮ����ˮ��Ӧ����NaOH������NaOH��������������Һ��Ũ��ƫ�ߣ���A��ȷ��
B.��ˮ�ܽ�NaOH���������ת������ƿ�ж��ݣ��ȵ���Һ���ƫ����ȴ�������С�������Ƶ���Һ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ���B��ȷ��
C.���ݺ���Һ����ڿ��ߣ����ý�ͷ�ιܽ������ˮ�������ᵼ���������ʵ������٣������Ƶ���ҺŨ��ƫ�ͣ���C����
D.����ʱ���ӿ̶���ʹ��Һ���ƫ�������Ƶ���ҺŨ��ƫ�ͣ���D����
E.��������ƽ����NaOH����ʱ���������⣬����NaOH��������������Һ��Ũ��ƫ�ߣ���E��ȷ��
F.����ǰ����ƿ��������ˮ�β�Ӱ���������ʵ�������Һ��������Բ�Ӱ��������Һ��Ũ�ȣ���F����
�ʴ�Ϊ��ABE��
��6���ܶ�Ϊ1.2g/cm3����������Ϊ36.5%��Ũ�������ʵ���Ũ��c=![]() =
=![]() =12mol/L������100mL3mol/L��ϡ���ᣬ���ù�ʽc1V1=c2V2���������ݵ�Ũ��������Ϊ25.0mL���ʴ�Ϊ��25.0��
=12mol/L������100mL3mol/L��ϡ���ᣬ���ù�ʽc1V1=c2V2���������ݵ�Ũ��������Ϊ25.0mL���ʴ�Ϊ��25.0��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��֤±�ص��������Ե����ǿ����ijС������ͼ��ʾװ�ý���ʵ��(�г���������ȥ���������Ѽ���)��
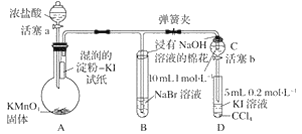
ʵ����̣�
��.���ɼУ�����a���μ�Ũ���ᡣ
��.��B��C�е���Һ����Ϊ��ɫʱ���н����ɼС�
��.��B����Һ�ɻ�ɫ��Ϊ�غ�ɫʱ���رջ���a��
��.����
��1����֤������������ǿ�ڵ��ʵ��������_________________________________________��
��2��B����Һ������Ӧ�����ӷ���ʽ��____________________________________________��
��3��Ϊ��֤���������ǿ�ڵ⣬�������IJ�����������________________________________��
��4��������ʵ���Ŀ����_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�ĵ��װ�ÿ�ʵ�ֵ͵�λ�¸�Ч����ԭCO2������˵������ȷ����(����)
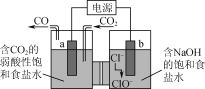
A.a��������ӵ�Դ�ĸ���
B.��������Na�����ҳ��������
C.b���ĵ缫��ӦʽΪCl����2e����H2O=ClO����2H��
D.���·��ÿת��1 mol���ӣ����ۿɴ���ԭ�����CO2����11.2 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪������AΪĿǰ����ʹ����㷺�Ľ�����������BΪ���д��Եĺ�ɫ���壬��������ת����ϵ��ա�
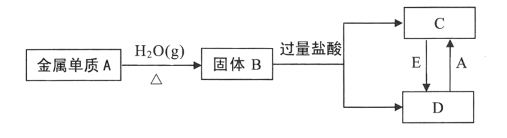
��1�����жϣ�A����ѧʽΪ___��B������Ϊ___��
��2��ʵ���Ҽ���C��Һ�е�������ʱ���ɼ�������������Һ�����Ȳ���___ɫ�������ó����ڿ�����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ___ɫ�������ӡ�����ת���Ļ�ѧ����ʽΪ___��
��3��ʵ���Ҽ���D��Һ�е�������ʱ��ͨ���ɵμ�___������Һ��Ϊ___ɫ�������ӡ�
��4����E��һ�ֻ���ɫ���嵥�ʣ���������___����C��D�����У���������___����
��5��д��D +A��C�����ӷ���ʽ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2V L Fe2(SO4)3��Һ�к���a g SO42����ȡ����ҺV L����ˮϡ����2V L����ϡ�ͺ���Һ��Fe3�������ʵ���Ũ��Ϊ
A.![]() mol��L��1B.
mol��L��1B.![]() mol��L��1C.
mol��L��1C.![]() mol��L��1D.
mol��L��1D.![]() mol��L��1
mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ����ຣʡ�������κ�ʢ��ʳ�Σ�������ʳ�ι�ϵ���У�ʳ�����ϰ���������ִ����Ĺ�ũҵ�����о�����Ҫ���á������к�Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ�Ϊ�˳�ȥ���������ʣ�������ʵ�鲽������ᴿ��
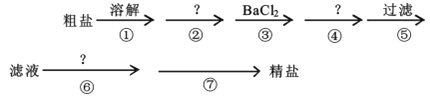
(1)�ܲ������Լ���___��
(2)�ڢ�������Ӧ�����ӷ���ʽΪ___��___��
(3)�ڢ߲��IJ�������___����Ҫ�IJ���������___��___��
(4)ͨ��������й��˺����Һ������SO42-�ѳ����IJ���������___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���ĺ��������������Ϊ��Ǧ���͵Ŀ��������������Է�������������100����C����������Ϊ68.2%����H����������Ϊ13.6%������Ϊ���������ش�
��1���û��������Է���������____________________��
��2��д���û�����ķ���ʽ___________________________��
��3�����û����ﲻ�����Ʒ�Ӧ����������������ͺ˴Ź���������ʾ�÷�������4��������д����ṹ��ʽ��_________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ��2L������X��Y��Z�������ʵ����ʵ�����n����ʱ�䣨t���仯��������ͼ��ʾ����ͼ������
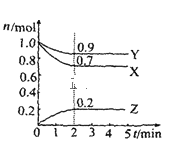
��1���÷�Ӧ�Ļ�ѧ����ʽΪ��______��
��2����Ӧ��ʼ��2min����Z��ʾ��ƽ����Ӧ����Ϊ��______��
��3������������˵��������Ӧ�ﵽ��ѧƽ��״̬����______������ţ���
A���������������ʵ�������ʱ��ı仯���仯
B����������ѹǿ����ʱ��ı仯���仯
C����λʱ����ÿ����3molX��ͬʱ����2molZ
D��������������������ʱ��ı仯���仯
��4�����ܱ������ͨ��amolX��g����bmolY��g����������ӦX��g��+Y��g���T2Z��g�������ı���������ʱ����Ӧ���ʻᷢ��ʲô�仯��ѡ����������������С����������������
�ٽ����¶ȣ�_____ �ں���ͨ�뺤����_____��ʹ�ô�����______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Na2CO3��NaHCO3�Ļ����27.4g���ȵ��������ٱ仯ʱ���ò�������21.2g����ԭ�������NaHCO3��Na2CO3������֮��Ϊ(����)
A.53��84B.1��1C.84��53D.42��53
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com