
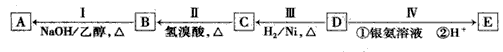
��ش��������⣺
(1)D�ķ���ʽΪ____ ��
(2)B�����������ŵ�����Ϊ___��
(3)��ķ�Ӧ����Ϊ____������ĸ��ţ���
a����ԭ��Ӧ
b���ӳɷ�Ӧ
c��������Ӧ
d����ȥ��Ӧ
(4)д�����з�Ӧ�Ļ�ѧ����ʽ�� I��____�� C��E����һ�������·�Ӧ����F��FΪ����ζ���л�������÷�Ӧ�Ļ�ѧ����ʽΪ____.
(5)A��������____��
(6)E�ж���ͬ���칹�壬����һ��ͬ���칹���ܷ���������Ӧ����������Ʒ�Ӧ�������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ____��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
| �� |
| �� |
| �� |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |






�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �Ҵ� |
| �� |
| �Ҵ� |
| �� |
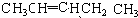
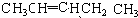


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�








�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���AΪ����������ͼ��������Է�������Ϊ70������ط�Ӧ����ͼ��ʾ������B��D��E�Ľṹ�о�����2����CH3�����ǵĺ˴Ź��������о�����4���塣
��ش�
��1��D�ķ���ʽΪ B�����������ŵ�����Ϊ ��
��2����ķ�Ӧ����Ϊ ������ĸ��ţ���
a����ԭ��Ӧ b���ӳɷ�Ӧ c��������Ӧ d����ȥ��Ӧ
��3��д����Ӧ��Ļ�ѧ����ʽ ��
��4����A������ͬ�����ŵ�ͬ���칹�壨������A������ �֣�
��5��E�ж���ͬ���칹�壬�����ܷ���������Ӧ�����������������������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ�������и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
�л���AΪ����������ͼ��������Է�������Ϊ70������ط�Ӧ����ͼ��ʾ������B��D��E�Ľṹ�о�����2����CH3�����ǵĺ˴Ź��������о�����4���塣
��ش�
��1��D�ķ���ʽΪ B�����������ŵ�����Ϊ ��
��2����ķ�Ӧ����Ϊ ������ĸ��ţ���
a����ԭ��Ӧ b���ӳɷ�Ӧ c��������Ӧ d����ȥ��Ӧ
��3��д����Ӧ��Ļ�ѧ����ʽ ��
��4����A������ͬ�����ŵ�ͬ���칹�壨������A������ �֣�
��5��E�ж���ͬ���칹�壬�����ܷ���������Ӧ�����������������������������ܷ�����ȥ��Ӧ����ṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com