

| �ζ����� | ���������ml�� | ���ռ������ml�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��1�� | 20.00 | 0.40 | 20.40 |
| ��2�� | 20.00 | 4.00 | 24.00 |
| ��3�� | 20.00 | 0.10 | 22.10 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��������Һ���Ƿ���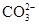 ���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ ���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ |
| B���Ӻ�I������Һ����ȡ�⣺��������ϡ������3%��H2O2��Һ�����þƾ���ȡ |
C��������Һ���Ƿ���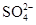 ���ȵμ�ϡ���ᣬ�ٵμ�BaCl2��Һ ���ȵμ�ϡ���ᣬ�ٵμ�BaCl2��Һ |
| D������NaCl��NH4Cl����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ��Ŀ�� | �� �� |
| | �Ƚ�ˮ���Ҵ����ǻ���Ļ�����ǿ�� | �ý����Ʒֱ���ˮ���Ҵ���Ӧ |
| | ��֤����Һ�к���SO42- | ���������ữ�����ᱵ��Һ |
| | ��֤����Һ�к���S2- | ȡ������Һ���ڴ���Ǧ��ֽ�Ͽ��Ƿ��� |
| | ֤��SO2����Ư���� | ��SO2ͨ������KMnO4��Һ�� |
| | �Ƚ�ȷ��ͭ��þ�Ľ������ǿ�� | ��Pt���缫���Mg(NO3)2��Cu( NO3)2���Һ |
| | ȷ��̼����Ԫ�طǽ�����ǿ�� | ��ͬ��ͬŨ��Na2CO3��Na2SiO3ˮ��Һ��PH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

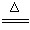


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
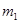 g�û�������Ʒ������ͼ1��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2+11O2
g�û�������Ʒ������ͼ1��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2 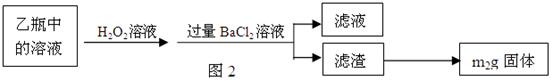
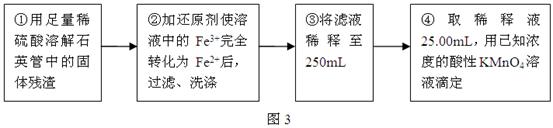
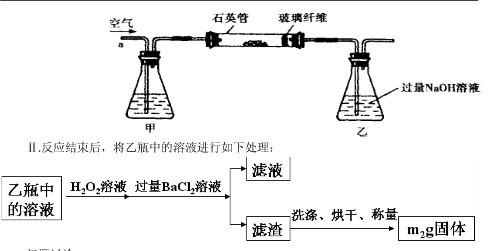
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
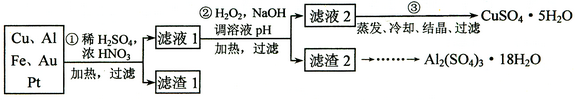
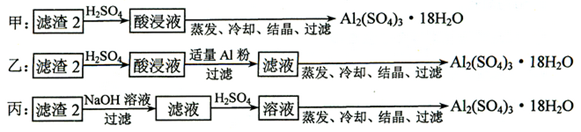
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com