
 ���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�
���ϳ�·�ߵ�һ���֡����������������¿����ģ�2���ٵľ����ٴ�����ϵ������Ȳ�ڼ״���һ����̼�����£���60�桢6 MPa�������ʻ�����һ���Ƶ�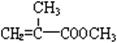 ���仯ѧ����ʽΪ�� ��
���仯ѧ����ʽΪ�� ��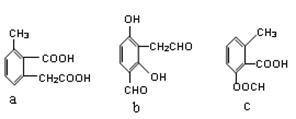
| A��װ����ǰ���ζ���δ�ñ���Һ��ϴ |
| B���ⶨ�������ʱ��ʼ���Ӷ���������Ӷ��� |
| C����ƿ�ñ�����������Һ��ϴ |
| D���ζ����������ὦ��ƿ�� |
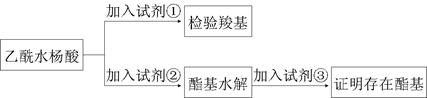
 ԭ�������ʴ�100%���ұ���������Ի�������Ⱦ
ԭ�������ʴ�100%���ұ���������Ի�������Ⱦ 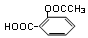 ��bc
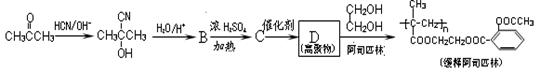
��bc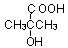 ��B������ȥ��Ӧ��õ�C��C�Ľṹ��ʽΪ
��B������ȥ��Ӧ��õ�C��C�Ľṹ��ʽΪ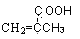 ,�߾���D�Ľṹ��ʽ�ǣ�
,�߾���D�Ľṹ��ʽ�ǣ�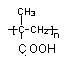 ����D���Ҷ�������˾ƥ�ַ�Ӧ���ɻ��Ͱ�˾ƥ�֣������Ƴ���˾ƥ�ֵĽṹ��ʽΪ
����D���Ҷ�������˾ƥ�ַ�Ӧ���ɻ��Ͱ�˾ƥ�֣������Ƴ���˾ƥ�ֵĽṹ��ʽΪ ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
| A��ȡ����Ӧ |
| B����ȥ��Ӧ |
| C��ˮ�ⷴӦ |
| D���кͷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
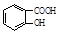 ����ش�
����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
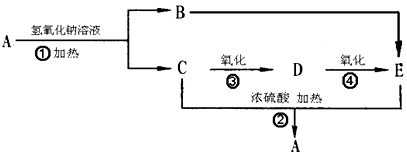
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
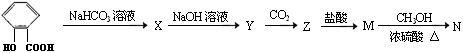
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
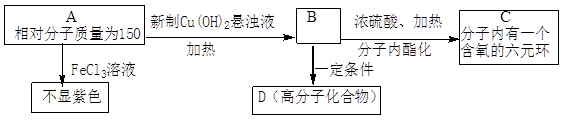
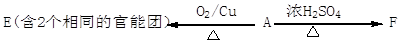
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com