
���� ��1�����ֵ��롢��Һ�д��ڵ���ƽ��ĵ����Ϊ������ʣ����������ȫ���������ˮ��Ĺ���������HNO2��������ʣ�
��2������кͺ���ʣ���ʹ��Һ��ʾ���ԣ���ʣ���ʹ��Һ��ʾ���ԣ�����ǿ��ǿ�����кͣ������Ӻ��������������ʵ�����ȣ���Һ��ʾ���ԣ�
��3������Ϊ������ʣ����ڵ���ƽ�⣬��ˮϡ��ʱ���ٽ�����ĵ��룻ˮ��������ʣ����ڵ���ƽ�⣬��������������������ˮ�ĵ���ƽ�⣻
��4����������HA��������NaA�Ļ����Һ�������м�����������ʱ����Һ������Ա仯���������ڼ�����ʱ����������ʣ������ʱ�������Σ���Һ�������ӻ�����������Ũ�ȱ仯������������ã�
��5�����ݶ�Ԫ��ĵ��뷽��ʽ֪��B2-ֻ������һ��ˮ�⣬��������غ�������
��6����25���£�ƽ��ʱ��Һ��c��NH4+��=c��Cl-��=0.005mol/L�����������غ��c��NH3��H2O��=��0.5a-0.005��mol/L�����ݵ���غ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ�NH3•H2O�ĵ��볣��Kb=$\frac{c��O{H}^{-}��•c��N{{H}_{4}}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$��
��� �⣺��1���ٳ�����HA��Һ��pHС��7��ֻ��֤����Ϊ�ᣬ����֤����Ϊ���ᣬ�ʴ���
����Һ�ĵ�����������Ũ�ȳ����ȣ���HNA��Һ������ʵ�飬���ݺܰ���ֻ��˵����Һ������Ũ�Ⱥ�С������˵��������ĵ���̶ȣ����Բ���֤��������Ϊ������ʣ��ʴ���
�۳�����NaA��Һ��pH����7��˵��NaAΪǿ�������Σ�������˵��HA��Ϊ���ᣬ����ȷ��
��0.1mol/L HA��Һ��pH=2.1��˵�����ȫ���룬��Һ�д��ڵ���ƽ�⣬������˵����Ϊ���ᣬ����ȷ��
�ݽ��������pH=2��HCl��HA�ֱ���������Zn��Ӧ���ų���H2���HA�࣮˵��HA��Ũ�ȴ�������ģ�����HA�����ᣬ����ȷ����
��pH=1��HA��Һϡ����100����pHԼΪ2.8˵�����д��ڵ���ƽ�⣬����Ϊ������ʣ�����ȷ��
��2���ٽ� pH=3��HA��Һ�뽫 pH=11��NaOH��Һ�������ϣ������������ᣬ��ʱ��ʣ�࣬��Һ��ʾ���ԣ���������ǿ�ᣬ��ʱ��Һ��ʾ���ԣ��ʴ�Ϊ��ac��
�ڣ��������ʵ���Ũ�ȵ�HA��Һ��NaOH��Һ�������Ϻ����������ᣬ��ʱǡ�÷�Ӧ���õ�ǿ�������Σ������������ˮ����ʾ���ԣ���A-+H2O?HA+OH-����Һ��ʾ���ԣ���������ǿ�ᣬ��ʱ��Һ��ʾ���ԣ�
�ʴ�Ϊ��bc��A-+H2O?HA+OH-��
��3��A������ǿ�ᣬ��������Һ��ϡ���������������ʵ�������5ml��10-3=V��10-4�����V=5Oml����10V��=V��������Ϊ���ᣬ��ˮϡ��ʱ���ٽ�����ĵ��룬������������������࣬ҪʹpH��ȻΪ4�������ˮӦ�ö�һЩ������10V�ף�V������A��ȷ��
B��pH=3�����У�����������ȫ����ˮ���������C��OH-����=$\frac{Kw}{c��{H}^{+}��}$=10-11mol/L��pH=4�����У�����������ȫ����ˮ���������C��OH-����=$\frac{Kw}{c��{H}^{+}��}$=10-10mol/L����10c��OH-����=c��OH-��������B����
C��������ǿ�ᣬ�ֱ���5mL pH=11��NaOH��Һ��Ӧ��ǡ�÷�������кͣ�����ǿ��ǿ���Σ�pHֵ��ȣ���Ϊ���ᣬ��Ӧ������ʣ�࣬����ʣ����Ũ�ȴ�����ǿ��pHС��������Һ��pH���ס��ң���C��ȷ��
D��ϡ��ǰ����������ձ���������һԪ������ʵ�����ȣ���������кͷ�Ӧ��֪�������������Ƶ����ʵ�����ȣ����ɵ����ε�Ũ�ȼ״����ң�����Ϊǿ�������pH��ȣ�����Ϊ���ᣬ���pH�����ң���D����
��ѡ��AC��
��4�����а�ˮ���Ȼ�淋Ļ����Һ�������м�����������ʱ����Һ������Ա仯���������ڼ����ʱ����������ʣ�������ʱ�������Σ������ڼ�����ʱ������NH3•H2O+H+?NH4++H2O�������ʱ������NH4++OH-?NH3•H2O����Һ�������ӻ�����������Ũ�ȱ仯������������ã�������ƵĻ��д���ʹ�������Һ���ʴ�Ϊ��AC��
��5����Na2B�д���ˮ��ƽ�⣺B2-+H2O=HB-+OH-��HB-�����һ��ˮ�⣬������Һ��û��H2B���ӣ����������غ��c��B2-��+c��HB-��=0.1mol•L-1��
�ʴ�Ϊ��c��HB-����
��6����25���£�ƽ��ʱ��Һ��c��NH4+��=c��Cl-��=0.005mol/L�����������غ��c��NH3��H2O��=��0.5a-0.005��mol/L�����ݵ���غ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ�NH3•H2O�ĵ��볣��Kb=$\frac{c��O{H}^{-}��•c��N{{H}_{4}}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$=$\frac{1{0}^{-7}��5��1{0}^{-3}}{0.5a-5��1{0}^{-3}}$=$\frac{1{0}^{-9}}{a-0.01}$��
�ʴ�Ϊ��$\frac{1{0}^{-9}}{a-0.01}$��
���� ���⿼����������ʵĵ��롢����Ũ�ȴ�С�ıȽϣ���ȷ������ʵ����ص��������غ㡢����غ�������غ����������ͬʱע�����Һ��ԭ����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����һԪ����HX��HY��HZ�������ƽ�ⳣ�����μ�С����ͬ���ͬpH�Ķ�Ӧ������Һ�У�ˮ�ĵ���ȴ�С��NaX��NaY��NaZ | |
| B�� | 0.1mol/LCH3COOH��Һ��0.1mol/LNaOH��Һ�������ϣ�������Һ�У�c��OH-��=c�� H+��+c��CH3COOH�� | |
| C�� | 0.1mol/LNaHS��Һ��0.1mol/LNaOH��Һ�������ϣ�������Һ�У�c��Na+����c�� S2-����c��HS-����c��OH-�� | |
| D�� | ��0.01mol/L��NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ�c��Na+��=c�� SO42-����c��NH4+����c��H+��=c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���뷴Ӧ�ʹ����ӵİٷ������ӣ���Ӧ���ʼӿ� | |
| B�� | ������μӵĻ�ѧ��Ӧ��������ѹǿ������С����������ӻ���ӵİٷ���ʹ��ѧ��Ӧ�������� | |
| C�� | �����¶�ʹ��ѧ��Ӧ�����������Ҫԭ���������˷�Ӧ������л���ӵİٷ��� | |
| D�� | ����Ӽ䷢������ײΪ��Ч��ײ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH��ͬ�Ģ�CH3COONa��NaHCO3��NaAlO2������Һ�е�c��Na+�����ڣ��ۣ��� | |
| B�� | ��0.5 mol/L��Na2CO3��Һ��amol/L��NaHCO3��Һ�������ϣ�c��Na+����c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| C�� | 10mL0.1mol/LCH3COOH��Һ��20mL0.1mol/LNaOH��Һ��Ϻ���Һ������Ũ�ȹ�ϵ��c��OH-��=c��H+��+c��CH3COO-��+2c��CH3COOH�� | |
| D�� | 25��ijŨ�ȵ�NaCN��Һ��pH=d����������ˮ�������c��OH-��=10-dmol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����¶ȣ�����Ӧ�������ӣ�����Ӧ���ʼ�С | |
| B�� | �ﵽƽ����뺤������Ӧ�������� | |
| C�� | �ﵽƽ��������¶Ȼ�����ѹǿ�������ڸ÷�Ӧƽ�������ƶ� | |
| D�� | �ﵽƽ�������A2��g����Ũ�ȣ�B2��ת�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
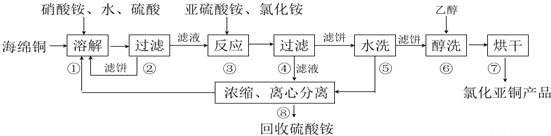
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Mg2+��Na+��SO42- | B�� | K+��H+��HCO3- | C�� | Cu2+��NO3-��SO42- | D�� | Ba2+��NO3-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com